��Ŀ����
16����ҵ���ö����������ƴ��ᣬ��Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��2C4H10+5O2$\stackrel{һ������}{��}$4CH3COOH+2H2O����58�ֶ���Ϊԭ����ȡ���ᣬ��1����������Ҫ��״���µĿ���2.8��105m3���������O2��N2����������ֱ�Ϊ0.2��0.8����ͬʱ����ˮ18�֣�
��2�������ɵĴ����ܽ������ɵ�ˮ�У����ô������������Ϊ86.96%��������λ��Ч���֣���
���� ��1����O2��Ҫ�����ʵ���Ϊxmol�����ݷ���ʽ�м������������ʵ�������������ڿ����еĺ���������Ҫ�����������
������ˮy�֣����ݷ���ʽ��������ˮ��������
��2�������ɴ���m�֣����ݷ���ʽ�������ɴ�����������������������Ķ�����㣮
��� �⣺��1����O2��Ҫ�����ʵ���Ϊxmol����
2 C4H10+5O2$\stackrel{һ������}{��}$4CH3COOH+2H2O��
58��2g 5mol
58��106 xmol
����x=$\frac{5��58��1{0}^{6}}{2��58}$=2.5��106
������Ҫ���������Ϊ$\frac{2.5��1{0}^{6}mol��22.4L/mol}{0.2}$=2.8��108L=2.8��105m3��
������ˮy�֣���
2 C4H10+5 O2$\stackrel{һ������}{��}$4CH3COOH+2H2O��
58��2g 18��2g
58�� y��
����y=$\frac{58��2��18}{58��2}$=18
�ʴ�Ϊ��2.8��105��18��
��2�������ɴ���m�֣���
2 C4H10+5 O2$\stackrel{һ������}{��}$4CH3COOH+2H2O��
58��2g 4��60g
58�� m��
����m=$\frac{58��4��60}{58��2}$=120
�ɣ�1���м����֪������ˮ18�֣�
���ɵĴ����ܽ������ɵ�ˮ�У����ô������������Ϊ$\frac{120��}{120��+18��}$��100%=86.96%��
�ʴ�Ϊ��86.96��
���� ���⿼����ݷ���ʽ�ļ��㣬�Ѷ��еȣ��������ϴ���ϸ�ļ��㣬ּ�ڿ���ѧ�������ݴ��������÷���ʽ���м��㣮

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Ư�۾� | B�� | ���� | C�� | �ռ� | D�� | ���� |
| A�� | 16.5g | B�� | 85g•mol-1 | C�� | 65 g | D�� | 55g•mol-1 |
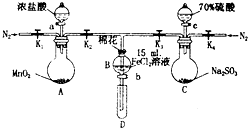 Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��
Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��ʵ����̣�
I�����ɼ�K1��K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
����a���μ�һ������Ũ���ᣬ��A���ȣ�
��B����Һ���ʱ��ֹͣ���ȣ��ر�K2��
��������b��ʹԼ2mL����Һ����D�Թ��У��������е����ӣ�
V����K3�ͻ���c������70%�����ᣬһ��ʱ���ر�K3��
���������Թ�D���ظ����̢�������B��Һ�е����ӣ�
��1������I��Ŀ�����ų�װ���еĿ�������ֹ���ţ�
��2������B����Һ�Ƿ���Fe2+�ķ���֮һ�ǣ�ȡ����B����Һ���Թ��У��μӼ��κ���+3����Ԫ�ص��������Һ���������ɫ������д���÷�Ӧ�����ӷ���ʽ3Fe2++2��Fe��CN��6��3-=Fe3��Fe��CN��6��2����
��3�������III��B�еĻ�ɫ��Һ��ͨ��H2S���壬��۲쵽�е���ɫ�������ɣ�д���÷�Ӧ�����ӷ���ʽ2Fe3++H2S�T2Fe2++2H++S����
��4��������ȡ��SO2ͨ�������ữ�ĸ��������Һ��ʹ��Һ��ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4��
��5���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���������ʾ�����ǵļ����һ�����ܹ�֤��������Cl2��Fe3+��SO2���Ǽף���ס������ҡ���������
| ���̢���B��Һ�к��е����� | ���̢���B��Һ�к��е����� | |
| �� | ��Fe3+��Fe2+ | ��SO42- |
| �� | ����Fe3+����Fe2+ | ��SO42- |
| �� | ��Fe3+��Fe2+ | ��Fe2+ |
| A�� | C60��һ�����͵Ļ����� | |
| B�� | C60��ʯī����̼��ͬ�������� | |
| C�� | C60����Ȼû�����Ӽ���������Ϊ���Ӿ��� | |
| D�� | C60��Է�������Ϊ720 g/mol |
| A�� | ������Ľṹʽ��H-Cl-O | |
| B�� | ����10�����ӵ���ԭ�ӵķ��ţ�${\;}_{8}^{18}$O | |
| C�� | S2-�Ľṹʾ��ͼ | |
| D�� | NH4Cl�ĵ���ʽ�� |
| A�� | ˮ | B�� | ��ˮ | C�� | ���� | D�� | �Ȼ��� |
��1�������й�����������ʵ��ж���ȷ����abcd��
a���������� b������������ c�����л�ԭ�� d���д̼�����ζ
��2����������ˮ�е���ķ���ʽΪH2SO3?H++HSO3-��HSO3-?H++SO32-�������ţ���
��3��������������Һ���뵽���ֲ�ͬ��Һ�е��������±���ʾ��
| ʵ����� | �Լ� | ���� |
| I | ����Ag2SO4��Һ | ������ɫ���� |
| II | 0.2mol•L-1CuCl2��Һ | ��Һ���̣������μӲ����ػ�ɫ���� |
| III | 0.1mol•L-1Al2��SO4��3��Һ | ��ʼ�����Ա仯�������μӲ�����ɫ���� |
�ھ����飬ʵ��II�е��ػ�ɫ�����в���SO42-������Cu+��Cu2+��SO32-���ƲⷴӦ�����Һ��һ���������ʵ��������������SO42-��Cl-����ѡ�ú��ʵ��Լ��ֱ���֤��Щ���ӣ�ȡ������Һ�������������ữ��μ��Ȼ�����Һ���а�ɫ�������ɣ�֤����SO42-����ȡ������Һ�������������ữ��μ����ᱵ��Һ�����ˣ���������Һ�еμ���������Һ���а�ɫ�������ɣ�֤����Cl-��
����֪��Al2��SO3��3��ˮ��Һ�в����ڣ������飬ʵ��III�еİ�ɫ��������SO42-���ð�ɫ������������ǿ�ᣬ��������ǿ�����ʹ����KMnO4��Һ��ɫ���Ʋ�����к��е�������ΪSO32-��OH-��

 ��
�� ��
�� ��
��
 ��
�� ���������֣�����д���֣�
���������֣�����д���֣�