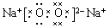��Ŀ����
3��������������ԭ�ͼ۸������ǣ���ʹ���������Ӵ�������Դ�����������Ҵ������ѽ���ʵ�û��Σ���1�������Ǻ�C15��C18��̼�⻯����Ļ�����Щ̼�⻯���������л����е����࣮
��2���Ҵ���ͭ�����������������£����Ա������е�����������X��X�Ľṹ��ʽ��CH3CHO��
��3���Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��Y���й����ŵ��������Ȼ�����Ũ���������£��Ҵ���Y��Ӧ������һ������ζ������W����ѧ����ʽΪCH3CH2OH+CH3COOH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��4��������ƿ��ɫҺ�壬�ֱ�ʢ��Y��W��ֻ��һ���Լ��Ϳ��Լ��𣬸��Լ�������ʯ����Һ��̼������Һ��
��5����ҵ��ͨ����ϩ��ˮ��һ�������·�Ӧ�Ƶ��Ҵ�����Ӧ�����Ǽӳɷ�Ӧ���ִ�ʯ�ͻ�������������������ϩ�ܱ������������ɻ�������
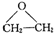 ���÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ2CH2=CH2+O2$��_{��}^{Ag}$
���÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ2CH2=CH2+O2$��_{��}^{Ag}$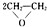 ��
��
���� ��1��̼�⻯�������C��HԪ�أ�
��2���Ҵ��ڴ������±���������XΪ��ȩ��
��3���Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��YΪ���ᣬWΪ����������
��4������������ԣ�����ʯ����Һ��̼������Һ���飻
��5����ϩ����̼̼˫��������ˮ�����ӳɷ�Ӧ�����Ҵ�����ϩ��������Ӧ���� ����������غ���ƽ����ʽ��
����������غ���ƽ����ʽ��
��� �⣺��1��̼�⻯�������C��HԪ�أ����������࣬�ʴ�Ϊ������
��2���Ҵ��ڴ������±���������XΪ��ȩ���ṹ��ʽΪCH3CHO���ʴ�Ϊ��CH3CHO��
��3���Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��YΪ���ᣬ���еĹ�����Ϊ�Ȼ���WΪ�����������Ҵ�������֮����Է���������Ӧ��
CH3CH2OH+CH3COOH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ���Ȼ���CH3CH2OH+CH3COOH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��4������������ԣ�����ʯ����Һ��̼������Һ���飬�ʴ�Ϊ��ʯ����Һ��̼������Һ��
��5����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����ϩ�ܱ������������ɻ������飬���������غ㶨�ɸ÷�ӦΪ2CH2=CH2+O2$��_{��}^{Ag}$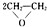 ��
��
�ʴ�Ϊ���ӳɷ�Ӧ��2CH2=CH2+O2$��_{��}^{Ag}$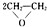 ��
��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע���Ҵ������ʼ�Ӧ�ã��ۺ��Խ�ǿ����Ŀ�ѶȲ���

�����ȶ��ԣ�H2O��HF��H2S��
��ԭ�Ӱ뾶��Na��Mg��O��
�����ԣ�H3PO4��H2SO4��HClO4��
�ܻ�ԭ�ԣ�I-��Br-��Cl-��
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
| A�� | ��̬��ԭ�Ӻ���۵��ӵĹ����ʾʽ�� | |
| B�� | HClO�ĽṹʽΪH-Cl-O | |
| C�� | �õ���ʽ��ʾNaCl���γɹ��̣� | |
| D�� | F-�Ľṹʾ��ͼ�� |
| ѡ�� | A | B | C | D |
| n��CO2����mol�� | 6 | 4 | 3 | 2 |
| n����������mol�� | 2 | 3 | 2 | 1 |
| A�� | A | B�� | B | C�� | C | D�� | D |
 �����⻯�������H+��������λ����ϣ�����Ľṹʽ
�����⻯�������H+��������λ����ϣ�����Ľṹʽ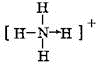 ��
�� ��1mol O22+�к��еĦм�Ϊ2mol��
��1mol O22+�к��еĦм�Ϊ2mol��