��Ŀ����
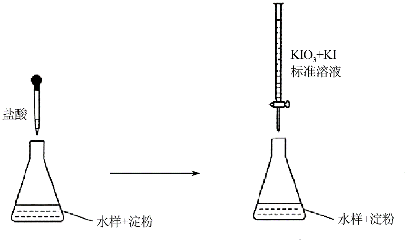
12�������£��ס��ҡ�����λͬѧ��ʵ��ȷ��ij��HA��������ʵķ����ֱ��ǣ��ף���pH��ֽ�ⶨ0.1moI/L HA��Һ��pH������֤��HA��������ʣ�
�ң��ٷֱ�ȡpH=l��HA��Һ��ϡ�����10.00mL���ټ�ˮϡ��Ϊ100mL��
�ڸ�ȡ��ͬ���������ϡ��Һ����������ͬʱ�ֱ���봿�Ⱥ���״��С����ͬ��п�������������۲�������֤��HA��������ʣ�
������������HA��Һ��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH����������������ݿ���˵��HA��������ʣ�
| ��� | NaOH/mol•L-1 | HA/mol•L-1 | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH��7 |
��2�����ҵķ����ĵڢٲ��У���Ҫ�õ��Ķ�����������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ�����У�ˮ�ĵ���̶ȵĴ�С��ϵ��c������ĸ����
a��HA��Һ��ˮ�ĵ���̶ȴ� b��ϡ������ˮ�ĵ���̶ȴ� c��������Һ��ˮ�ĵ���̶���ͬ
��3���ҵķ����У�˵��HA��������ʵ���Ҫ������B������ĸ����
A��װϡ������Թ��зų�H2�����ʿ�
B��װHA��Һ���Թ��зų�H2�����ʿ�
C�������Թ��в������������һ����
��4�����ķ����У���Ţ��е�c�������������������=����0��l���û��Һ�е�����Ũ�ȣ�c��Na+��=�����������������=����c��A-����
��5�����ķ����У���Ţ۵����ݱ����������Һ��HA�ĵ���̶ȱ�NaA��ˮ��̶ȣ�ǿ���ǿ��������������ȷ��������
���� ��1��������ʵĵ����ǿ���ģ�������ȫ���룻������������Ȱ�һС��pH��ֽ���ڱ��������Ƭ�ϣ����ò�����պԦ��Һ������ֽ���в�������ɫ�������ɫ���Ա�ȷ����Һ��pH��
��2����ȷȡpH=1��HA��Һ��ϡ�����10.00mL������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ�����У�˵��������Ũ����ȣ�����ˮ�����ӻ����õ�����������Ũ����ȣ�����������Һ��ˮ�Ķ����̶���ͬ��
��3��������ˮ��Һ�ﲿ�ֵ��룬����������Ũ��С����Ũ�ȣ����п��Ӧʱ������������Ӧ���ʺ�������Ũ�ȳ����ȣ��������������ķ�Ӧ����ȷ�����ǿ����
��4������Ϊǿ�ᣬ�������Ũ�Ȼ��ʱpH=7����HAΪ���ᣬ�������Ũ�Ȼ����Һ��pH����7����Ϊ��֤pH=7��Ӧʹ��Ũ�ȴ���0.1mol/L������ϵ���غ��������Ũ�ȹ�ϵ��
��5���ɢ���ʵ������֪����Ϻ�ΪHA��NaA�Ļ��Һ��pH=7����ĵ�������ε�ˮ�⣮
��� �⣺��1�����������ˮ��Һ�в��ֵ��룬��HAΪ���ᣬ0.1 mol/L HA��Һ��c��H+����0.1 mol/L����Һ��pH��1���ײⶨ��ҺPH�ľ�������������Ȱ�һС��pH��ֽ���ڱ��������Ƭ�ϣ����ò�����պԦ��Һ������ֽ���в�������ɫ�������ɫ���Ա�ȷ����Һ��pH��
�ʴ�Ϊ�������Ȱ�һС��pH��ֽ���ڱ��������Ƭ�ϣ����ò�����պԦ��Һ������ֽ���в�������ɫ�������ɫ���Ա�ȷ����Һ��pH��
��2�����ҵķ����ĵڢٲ��У�ȷ��ȡ10.00mL������Һ��Ҫ�õ��Ķ�����������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ������������Ũ����ͬ������ˮ�ĵ���̶ȵ���ͬ��
�ʴ�Ϊ����ʽ�ζ��ܣ�c��
��3���������Խϡ����̶�Խ��ϡ����ͬ����������������Ũ�ȱ仯С��������Ũ�ȴ��ҵķ����У�˵��HA��������ʵ���Ҫ������װHA��Һ���Թ��зų�H2�����ʿ죬
�ʴ�Ϊ��B��
��4�����ķ�����ͨ����Ţٵ�����֤��HAΪ���ᣬ�����������Ũ�Ȼ����Һ�ʼ��ԣ���Ţ��г����ԣ�����c��0��l�����ݵ���غ㣬�û��Һ�е�����Ũ�ȣ�c��Na+��=c��A-����
�ʴ�Ϊ������=��
��5�����ķ����У���Ţ�ʵ�����ҺΪ��Ũ�ȵ�NaA��HA�Ļ��Һ��NaAˮ��ʼ��ԡ�HA��������ԣ�ʵ�����ݱ�����Һ�����ԣ������Һ��HA�ĵ���̶ȱ�NaA��ˮ��̶�ǿ���ʴ�Ϊ��ǿ��
���� ���⿼��������ʵĵ��룬Ϊ��Ƶ���㣬���ؿ���ѧ�������ж���������ȷ������ʵ����ص㼰������ʵ���Ӱ�������ǽⱾ��ؼ���ע�⣺��Ӱ��ˮ����̶���������Ũ���йء������ǿ���أ�Ϊ�״��㣮

��1����������Ӧ�ﵽƽ�����CCl4��ȡI2�����¶Ȳ��䣬��ѧ��Ӧ���ʼ�������������������䡱����v��������v���棩�������������=������
��2���÷�Ӧ������Ӧ���ʺ�Fe3+��I-��Ũ�ȹ�ϵΪv=kcm��I-��•cn��Fe3+����kΪ��������
| c��I-��/mol•L-1 | c��Fe3+��/mol•L-1 | v/mol•L-1•s-1 | |
| �� | 0.20 | 0.80 | 0.032k |
| �� | 0.60 | 0.40 | 0.144k |
| �� | 0.80 | 0.20 | 0.128k |
A��m=1��n=1 B��m=1��n=2 C��m=2��n=1 D��m=2��n=2
I-Ũ�ȶԷ�Ӧ���ʵ�Ӱ����ڣ��������������=����Fe3+Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
| A�� | ��״���£�2.24L����������������Ӧ��ת�Ƶ�����Ϊ0.2NA | |
| B�� | ��״���£�2.24L NO2������������Ϊ3.9 NA | |
| C�� | 7.8gNa2S��Na2O2�Ļ�����к��е���������������0.1 NA | |
| D�� | ���³�ѹ�£���0.1mol����ͨ��ˮ�з�����ѧ��Ӧ��ת�Ƶ�����Ϊ0.1 NA |
| A�� | 2KBr+Cl2=2KCl+Br2 | B�� | CaCO3=CaO+CO2�� | ||
| C�� | SO3+H2O=H2SO4 | D�� | MgCl2+2NaOH=Mg��OH��2��+NaCl |
| A�� | ú�����������Ȼ����ʯ�����л������� | |
| B�� | ���û�ʯȼ��ȼ�շų�������ʹ�ֽ���������������ܿ������о����� | |
| C�� | ��ѧ��Դ�ŵ硢ֲ�������ö��ܷ�����ѧ�仯��������������ת�� | |
| D�� | ����ѧ�����жϿ���ѧ���ų������������γɻ�ѧ�������յ���������Ӧ���� |
| A�� | ��ҵ������������ˮ�ࡢƯ�۾���Ҫ��ʯ��ʯΪԭ�� | |
| B�� | �û���̿Ϊ�ǽ���ɫ���ô�������Ư��ֽ����ԭ����ͬ | |
| C�� | ����ӻ�������֬��������ˮ��Ϊ��������͵�С���Ӳ��ܱ����� | |
| D�� | ˾ĸ�춦����Զ���װ塢�л�������ԭ�������ںϽ� |