��Ŀ����
��֪HA��һ�����ᣮ��ش��������⣺
��1������һ�ֺ���HA��������NaA����Һ��
����ɸ���Һ���������� ��
���������Һ�м�����������ʱ��������Ӧ�����ӷ���ʽ�� ��
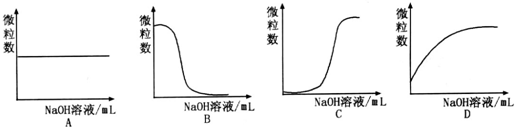
���������Һ����μ���NaOH��Һʱ������ͼ���ܱ�ʾA-������Ŀ�仯���Ƶ��� ������ĸ����
��2���ֽ�1���0.04mol?L-1 HA��Һ��1���0.02mol?L-1 NaOH��Һ��ϣ��õ�2��������Һ��
�����û��Һ�Լ��ԣ�����c��A-�� 0.01mol?L-1�����������=����������
�����û��Һ�����ԣ�����Һ���������ӵ�Ũ���ɴ�С��˳���� ��
��1������һ�ֺ���HA��������NaA����Һ��
����ɸ���Һ����������
���������Һ�м�����������ʱ��������Ӧ�����ӷ���ʽ��
���������Һ����μ���NaOH��Һʱ������ͼ���ܱ�ʾA-������Ŀ�仯���Ƶ���

��2���ֽ�1���0.04mol?L-1 HA��Һ��1���0.02mol?L-1 NaOH��Һ��ϣ��õ�2��������Һ��
�����û��Һ�Լ��ԣ�����c��A-��
�����û��Һ�����ԣ�����Һ���������ӵ�Ũ���ɴ�С��˳����
���㣺���������ˮ��Һ�еĵ���ƽ��,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��
��������1������Һ�д���������ӵ�ˮ��ƽ�⡢ˮ�ĵ���ƽ�⡢����εĵ��룬�ݴ��ж���Һ�д��ڵ�����
������������ܺ�ǿ�ᷴӦ�������
��������Һ�м�����������ʱ���������ƺ��ᷴӦ�����Σ��ٽ���ĵ��룻
��2���ֽ�1���0.04mol?L-1 HA��Һ��1���0.02mol?L-1 NaOH��Һ��ϣ���Һ�е������ǵ����ʵ���Ũ�ȵ�HA��NaA��
�����û��Һ�Լ��ԣ���A-ˮ��̶ȴ���HA�ĵ���̶ȣ�
�����û��Һ�����ԣ���A-����̶ȳ̶ȴ���ˮ��̶ȣ�
������������ܺ�ǿ�ᷴӦ�������
��������Һ�м�����������ʱ���������ƺ��ᷴӦ�����Σ��ٽ���ĵ��룻
��2���ֽ�1���0.04mol?L-1 HA��Һ��1���0.02mol?L-1 NaOH��Һ��ϣ���Һ�е������ǵ����ʵ���Ũ�ȵ�HA��NaA��
�����û��Һ�Լ��ԣ���A-ˮ��̶ȴ���HA�ĵ���̶ȣ�
�����û��Һ�����ԣ���A-����̶ȳ̶ȴ���ˮ��̶ȣ�
���
�⣺��1����HAΪ������ʣ����ڵ���ƽ�⣬ˮ��������ʣ����ڵ���ƽ�⣬NaAΪǿ����ʣ���ȫ���룬������Һ�д��ڵ�����H2O��HA��H+��OH-��A-��Na+��
�ʴ�Ϊ��H2O��HA��H+��OH-��A-��Na+��
���������Һ�м�����������ʱ���Ȼ��������������Ӻ�������ӷ�Ӧ����HA�������ӷ�Ӧ����ʽΪA-+H+?HA���ʴ�Ϊ��A-+H+?HA��
���������Һ�м���NaOH��Һʱ�����������Ӻ������ӷ�Ӧ����ˮ���Ӷ��ٽ�HA�ĵ��룬������Һ�����������Ŀ������ȫ��Ӧʱ���������Ũ�����ѡD��
��2���ֽ�1���0.04mol?L-1 HA��Һ��1���0.02mol?L-1 NaOH��Һ��ϣ���Һ�е������ǵ����ʵ���Ũ�ȵ�HA��NaA�������ʵ���Ũ�ȶ���0.01mol/L��
�����û��Һ�Լ��ԣ���A-ˮ��̶ȴ���HA�ĵ���̶ȣ�����c��A-����0.01mol?L-1���ʴ�Ϊ������
�����û��Һ�����ԣ���A-����̶ȳ̶ȴ���ˮ��̶ȣ���Һ�����ԣ���c��H+����c��OH-������Һ�д��ڵ���غ�c��H+��+c��Na+��=c��A-��+c��OH-��������c��Na+����c��A-������ĵ���̶Ƚ�С������c��H+����c��Na+��������Һ������Ũ�ȴ�С˳����c��A-����c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��A-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��H2O��HA��H+��OH-��A-��Na+��
���������Һ�м�����������ʱ���Ȼ��������������Ӻ�������ӷ�Ӧ����HA�������ӷ�Ӧ����ʽΪA-+H+?HA���ʴ�Ϊ��A-+H+?HA��
���������Һ�м���NaOH��Һʱ�����������Ӻ������ӷ�Ӧ����ˮ���Ӷ��ٽ�HA�ĵ��룬������Һ�����������Ŀ������ȫ��Ӧʱ���������Ũ�����ѡD��
��2���ֽ�1���0.04mol?L-1 HA��Һ��1���0.02mol?L-1 NaOH��Һ��ϣ���Һ�е������ǵ����ʵ���Ũ�ȵ�HA��NaA�������ʵ���Ũ�ȶ���0.01mol/L��
�����û��Һ�Լ��ԣ���A-ˮ��̶ȴ���HA�ĵ���̶ȣ�����c��A-����0.01mol?L-1���ʴ�Ϊ������
�����û��Һ�����ԣ���A-����̶ȳ̶ȴ���ˮ��̶ȣ���Һ�����ԣ���c��H+����c��OH-������Һ�д��ڵ���غ�c��H+��+c��Na+��=c��A-��+c��OH-��������c��Na+����c��A-������ĵ���̶Ƚ�С������c��H+����c��Na+��������Һ������Ũ�ȴ�С˳����c��A-����c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��A-����c��Na+����c��H+����c��OH-����
���������⿼��������ˮ���������ʵĵ��룬��������ˮ��ƽ���������ʵ���ƽ��ȷ�������Һ�д��ڵ��������ݻ����Һ������Խ�ϵ���غ�ȷ����Һ������Ũ�ȴ�С���״����ǣ�2����ע������ʱ����Һ���������һ�����������ʵ�Ũ�ȶ����ͣ�Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A��2013��0�����������ļ���Сʱ����������Ⱦָ���������Ⱦ����Ϊ�ض���Ⱦ����Ҫ��ȾΪPM2.5��Ⱦ�������̻������ȼ�ţ��ɼ���PM2.5��Ⱦ |
| B������֮�����ܼӿ췴Ӧ���ʣ�����Ϊ�����ܽ��ͷ�Ӧ�Ļ�� |
| C��������ˮ��Һ��ˮ���Ե��磬��˰����ǵ���� |
| D������ȼ�պ���ȼ�����γ��������Ҫԭ�� |
���в����У�����ȷ���ǣ�������
| A������մ������Ӧ�����þƾ�ϴ�� |
| B��ȼ�ŵľƾ��ƴ����Ӧ��ˮ���� |
| C��ʵ�����ҽ���ʯ�ͷ�������ϩʵ��ʱ�������ڷ�Ӧ���м����������Ƭ |
| D����ȼ���顢��ϩ�ȿ�ȼ������ǰ�����ȼ����䴿�� |
���и������ӣ���ǿ������Һ�п��Դ���������ǣ�������
| A��K+��Ca2+��HCO3-��Cl- |
| B��Ba2+��Na+��AlO2-��NO3- |
| C��NH4+��Na+��NO3-��CO32- |
| D��Mg2+��Na+��Cl-��SO42- |
 Na2SO3��SO2����ѧ���������ʣ�
Na2SO3��SO2����ѧ���������ʣ� I��������Ԫ��X��Y��Z��W��Ԫ�����ڱ������λ����ͼ��ʾ������Y������������������������ȣ���Ҫ��ش��������⣺
I��������Ԫ��X��Y��Z��W��Ԫ�����ڱ������λ����ͼ��ʾ������Y������������������������ȣ���Ҫ��ش��������⣺