��Ŀ����
11���ۺ���������[Fe2��OH��n��SO4����3-0.5n��]m��n��2��m��10����ƾ�������һ�ָ�Ч����������ij��ѧѧϰС�����ⶨ����������Ʒ��Һ����Ԫ���������������������Ʊ��ۺ�����������Ƶ�ʵ��������£�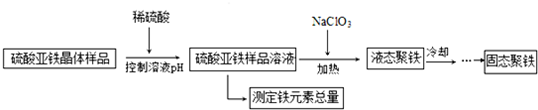
��ش��������⣺
��1��ʵ����Ҫ��Լ100mL��1��9���ᣨŨ�������ܼ�ˮ������ȣ������Ƹ�����ʱ��Ҫ�IJ��������ǣ����������ձ�����Ͳ�����ƹ���������Ͳ��ȡ90mLˮ�����ձ��У�����ȡ10mLŨ�����ز������������뵽�ձ��У��ӱ߽��裮
��2�����������Լ�������ˮ��ϡ���ᡢKSCN��Һ��K3[Fe��CN��6]��Һ��������ˮ
ijͬѧͨ��ʵ���ж��������������Ѳ��ֱ��ʣ�����Ƶ�ʵ���ǣ�������������������ϡ�����У�������ˮϡ�ͣ�ȡ������Һ��һ����Һ�м���KSCN��Һ����Һ��ɺ�ɫ֤���ѱ��ʣ���һ����Һ�м���K3[Fe��CN��6]��Һ��������ɫ����֤�����ֱ��ʣ��뽫�����������۲�����������
��3�����������Ƶ�ij�־���[Fe2��OH��4SO4]4�Ļ�ѧ����ʽ��24FeSO4+4NaClO3+36H2O=3[Fe2��OH��4SO4]4+4NaCl+12H2SO4��
��4���ⶨ����������Ʒ��Һ����Ԫ��������ʵ�����£�ȷ��ȡ5.00mL��Һ�����������ƿ�У���������H2O2���������һ��ʱ�䣬�������KI����ַ�Ӧ������0.5000mol/LNa2S2O3����Һ�ζ����յ㣬���ı���Һ20.00mL��
��ʾ��2Fe3++2I-=2Fe2++I2�� I2+2S2O32-=2I-+S4O62-����ɫ��
�ζ�ʱѡ�õ�ָʾ���ǵ�����Һ������������Ʒ��Һ����Ԫ��������112g/L��ʵ������У���ʡ�Բ��衰�������һ��ʱ�䡱���Եζ�����к�Ӱ�죬��˵���жϵ�����ƫ�ߣ�������H2O2��KI������I2��ʹNa2S2O3����Һ����ƫ���²ⶨ����Ԫ������ƫ�ߣ�
���� ��1��100mL��1��9������ҪŨ����10mL��ˮ90mL����ȡ90mLˮ�����ձ��У�����ȡ10mLŨ�����ز������������뵽�ձ��У��ӱ߽��裻
��2���������������Ѳ��ֱ��ʣ�������������������Ϊ�����������������Ļ�����K3[Fe��CN��6]��Һ�����������ӣ���KSCN��Һ���������ӣ�
��3��������ͼ��֪������������NaClO3�����������õ�[Fe2��OH��4SO4]4����Ԫ�ر���ԭ����NaCl�������غ��֪��������H2SO4��
��4���ⵥ������������ʾ��ɫ����ѡ����Ϊָʾ�������ⵥ����ȫ��Ӧ����Һ����ɫ��Ϊ��ɫ��
�ڸ��ݷ�Ӧ��2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-���ɵù�ϵʽFe3+��S2O32-��Ȼ����ݹ�ϵʽ����������ӵ����ʵ������ټ�����Ԫ�ص��ܺ�����
�������Ŀ���dz�ȥ����H2O2������˫��ˮ���Խ�I-����ΪI2����������S2O32-��ƫ�࣮
��� �⣺��1��100mL��1��9������ҪŨ����10mL��ˮ90mL������Ͳ��ȡ90mLˮ�����ձ��У�����ȡ10mLŨ�����ز������������뵽�ձ��У��ӱ߽��裬
�ʴ�Ϊ���ձ�����Ͳ������Ͳ��ȡ90mLˮ�����ձ��У�����ȡ10mLŨ�����ز������������뵽�ձ��У��ӱ߽��裻
��2���������������Ѳ��ֱ��ʣ�������������������Ϊ�����������������Ļ������鲿�ֱ��ʵķ���Ϊ��������������������ϡ�����У�������ˮϡ�ͣ�ȡ������Һ��һ����Һ�м���KSCN��Һ����Һ��ɺ�ɫ֤���ѱ��ʣ���һ����Һ�м���K3[Fe��CN��6]��Һ��������ɫ����֤�����ֱ��ʣ�
�ʴ�Ϊ��ȡ������Һ��һ����Һ�м���KSCN��Һ����Һ��ɺ�ɫ֤���ѱ��ʣ���һ����Һ�м���K3[Fe��CN��6]��Һ��������ɫ����֤�����ֱ��ʣ�
��3��������ͼ��֪������������NaClO3�����������õ�[Fe2��OH��4SO4]4����Ԫ�ر���ԭ����NaCl�������غ��֪��������H2SO4����Ӧ����ʽΪ��24FeSO4+4NaClO3+36H2O=3[Fe2��OH��4SO4]4+4NaCl+12H2SO4��
�ʴ�Ϊ��24FeSO4+4NaClO3+36H2O=3[Fe2��OH��4SO4]4+4NaCl+12H2SO4��
��4���ⵥ������������ʾ��ɫ����ѡ����Ϊָʾ�������������һ�������������Һʱ����ɫ��ʧ�Ұ���Ӳ���ɫ˵����Ӧ�����յ㣬
�ɹ�ϵʽFe3+��S2O32-����n��Fe3+��=n��S2O32-��=0.5000mol/L��0.02L=0.01mol����Ԫ���ܺ���Ϊ��$\frac{56g/mol��0.01mol}{0.005L}$=112g/L��
������H2O2��KI������I2��ʹNa2S2O3����Һ����ƫ���²ⶨ����Ԫ������ƫ�ߣ�
�ʴ�Ϊ��������Һ��112��ƫ�ߣ�������H2O2��KI������I2��ʹNa2S2O3����Һ����ƫ���²ⶨ����Ԫ������ƫ�ߣ�
���� ��������������������Ϊ���壬������Һ���ơ�ʵ�鷽����ơ�İ������ʽ����д�����ʺ����ⶨ�ȣ���֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

 53���ò�ϵ�д�
53���ò�ϵ�д�| A�� | ������Һ���������Ṳ�Ⱥ�NaOH��Һ�����ԣ��ټӵ�ˮ����������˵��������ˮ����ȫ | |
| B�� | ��ȡһ�����ѳ����NaOH�����һ�������Һ��ȡ����Һ�ζ�δ֪Ũ�����ᣬ������ⶨŨ��ƫ�� | |
| C�� | �����豸������Ļ��֣����ö�����̼�����Ȼ�̼��������Ҳ������ĭ�������� | |
| D�� | ����������Һ�м��������ˮ����Һ�Ȼ��Ǻ���壬˵������������������ |
����ȥ��Ӧ��ȡ����Ӧ�ۼӳɷ�Ӧ��ˮ�ⷴӦ��
| A�� | �٢� | B�� | �٢ڢ� | C�� | �ڢ� | D�� | �٢ڢۢ� |
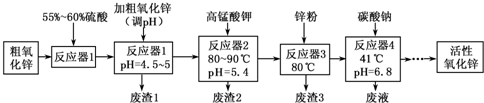
| Fe��OH��2 | Fe�� OH��3 | Cu��OH��2 | Zn��OH��2 | Mn��OH��2 | |
| ��ʼ������pH | 7.5 | 2.2 | 5.2 | 6.4 | 8.6 |
| ������ȫ��pH | 9.0 | 3.2 | 6.7 | 8.0 | 10.1 |
��1��������1������Ҫ�ɷ���Fe�� OH��3��
��2����ɡ���Ӧ��2���з�Ӧ֮һ�����ӷ���ʽ����MnO-4+��Mn2++��2H2O=��MnO2��+��H+
��3��պȡ����Ӧ��2���е���Һ���ڵ��۵⻯����ֽ�ϣ�����۲쵽��ֽ������˵��KMnO4������
��4��������2���������MnO2��������ȡMnO����֪��
2MnO2��s��+C��s���T2MnO��s��+CO2��g����H=-174.6kJ•mol-1
C��s��+CO2��g���T2CO��g����H=+283.0kJ•mol-1
��д��MnO2��s����CO��g����Ӧ��ȡMnO��s�����Ȼ�ѧ����ʽ��MnO2��s��+CO��g��=MnO��s��+CO2��g����H=-228.8kJ/mol��
��5������Ӧ��3���м���п�۵������ǵ�����ҺpH����ȥ��Һ��Cu2+��
��6������Ӧ��4���õ��ķ�Һ�У����е���Ҫ���ӳ���Na+�⣬����K+��SO42-��
��7���ӡ���Ӧ��4���о����˵Ȳ����õ���ʽ̼��п��ȡ��ʽ̼��п3.41g����400��450���¼��������أ��õ�ZnO 2.43g�ͱ�״����CO2��0.224L����ʽ̼��п�Ļ�ѧʽZnCO3•2Zn��OH��2•H2O��
| A�� | 3.2gO2��O3��ɵĻ�����к��е�������Ϊ1.6NA | |
| B�� | ����Ӧ6HCl+KClO3�TCl2��+KC1+3H2O��71gC l2����ʱ��ת�Ƶ�����ĿΪ2NA | |
| C�� | 1molC2H4�����к����õ��ӶԵ�����Ϊ5NA | |
| D�� | 0.1mol�Ҵ������������ַ�Ӧ�����ɵ�ˮ������ĿΪ0.1NA |

 ��R1��R2������������ԭ�ӣ�
��R1��R2������������ԭ�ӣ� ij������ȤС��ⶨijδ֪Ũ�ȵ�NaOH��Һʱ�����ǵIJⶨ���̴������£�
ij������ȤС��ⶨijδ֪Ũ�ȵ�NaOH��Һʱ�����ǵIJⶨ���̴������£�



 ������Ϊ�ڶ��ױ���
������Ϊ�ڶ��ױ���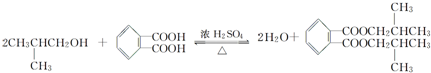 ��
��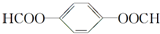 �� 1molX��NaOH��Һ���ȷ�Ӧ���������4molNaOH��
�� 1molX��NaOH��Һ���ȷ�Ӧ���������4molNaOH��