��Ŀ����
ʵ���ǻ�ѧ�о���һ����Ҫ�ֶΣ�������ͼ��ʾA��G���������������Ҫ����գ�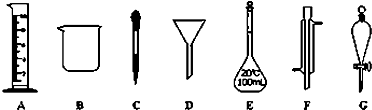
��1��д���������ƣ�E ��G ��
��2������ʵ��������õ�����G���� ��ѡ�����ĸ����ͬ����
a������ˮ��CCl4�Ļ���� b������ˮ�;ƾ��Ļ���� c������ˮ����ɰ�Ļ����
��3��ʵ��������������������Ϊ36.5%���ܶ�Ϊ1.19g/cm3��Ũ��������95mL.5mol/L��������Һ��
�����й�������E��ʹ�÷����У���ȷ���� ��
a��ʹ��ǰӦ����Ƿ�©Һ b��ʹ��ǰ������
c�������������ʷ�Ӧ���ܽ������ d������Һ��ֱ��ת�Ƶ�����ƿ��
������Ũ��������ʵ���Ũ��Ϊ mol/L����Ҫ��ȡ��Ũ����� mL��

��1��д���������ƣ�E
��2������ʵ��������õ�����G����
a������ˮ��CCl4�Ļ���� b������ˮ�;ƾ��Ļ���� c������ˮ����ɰ�Ļ����
��3��ʵ��������������������Ϊ36.5%���ܶ�Ϊ1.19g/cm3��Ũ��������95mL.5mol/L��������Һ��
�����й�������E��ʹ�÷����У���ȷ����
a��ʹ��ǰӦ����Ƿ�©Һ b��ʹ��ǰ������
c�������������ʷ�Ӧ���ܽ������ d������Һ��ֱ��ת�Ƶ�����ƿ��
������Ũ��������ʵ���Ũ��Ϊ
���㣺���ʵķ��롢�ᴿ�Ļ�������ѡ����Ӧ��,��Һ������
ר�⣺ʵ����
��������1�����������Ľṹ�ص��ж����������ƣ�
��2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����
��3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ������������;��
�ڸ���c=
����Ũ�����Ũ�ȣ��������ǰ�����ʵ����ʵ�����ȼ���Ũ���������
��2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����
��3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ������������;��
�ڸ���c=
| 1000��w |
| M |
���
�⣺��1����������ͼ�ο�֪EΪ����ƿ��GΪ��Һ©�����ʴ�Ϊ������ƿ����Һ©����
��2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����ӦΪa���ʴ�Ϊ��a��
��3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ����ʹ��ǰҪ����Ƿ�©ˮ������Ҫ��ɣ��Ҳ��ܽ��ȵ���Һת�Ƶ�����ƿ�У��ʴ�Ϊ��ac��
��Ũ�����Ũ��Ϊc=
=11.9mol/L������95mL.5mol/L��������Һ����Ҫ��ȡ��Ũ�����Ϊ
=0.042L=42mL��
�ʴ�Ϊ��11.9��42��
��2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����ӦΪa���ʴ�Ϊ��a��
��3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ����ʹ��ǰҪ����Ƿ�©ˮ������Ҫ��ɣ��Ҳ��ܽ��ȵ���Һת�Ƶ�����ƿ�У��ʴ�Ϊ��ac��
��Ũ�����Ũ��Ϊc=
| 1000��1.19��36.5% |
| 36.5 |
| 0.1L��5mol/L |
| 11.9mol/L |
�ʴ�Ϊ��11.9��42��
���������⿼���Ϊ�ۺϣ��漰���ʵķ����ᴿ��������ʹ�á���Һ�����Ƶ�֪ʶ�Ŀ��飬����ѧ���Ļ���ʵ�����������ʵ�����֪ʶ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ
�����и���Һ�У�һ���ܴ���������������ǣ�������
| A��������pH=7����Һ�У�Fe3+��Na+��Cl-��SCN- |
| B��������c��OH-��/c��H+��=1014����Һ�У�K+��Mg2+��Cl-��Br- |
| C�����д���HCO3-�ij�������Һ�У�K+��C6H5O-��Cl-��Na+ |
| D�������������Ӧ�ų���������Һ�У�K+��NO3-��Cl-��Fe2+ |
���ı�����һ������������ͨ�������ӵİٷ�������߷�Ӧ���ʵ��ǣ�������
| A����ѹ | B������ | C������ | D������ |
��һ���Լ����ܼ������ȩ�����ᡢ�Ҵ������ᣬ���Լ��ǣ�������
| A��������Һ |
| B�����Ƶ�Cu��OH��2 ����Һ |
| C����ˮ |
| D��̼������Һ |
 ͭ�����ᷴӦ�����ʵ����Ĺ�ϵ��ͼ��ʾ���������ʾ����ͭ�����ʵ������������ʾ������������ʵ���������ͼ�и����߱�ʾ���з�Ӧ��
ͭ�����ᷴӦ�����ʵ����Ĺ�ϵ��ͼ��ʾ���������ʾ����ͭ�����ʵ������������ʾ������������ʵ���������ͼ�и����߱�ʾ���з�Ӧ��