��Ŀ����
5����������һ�����͵����ǽ������ϣ����㷺Ӧ���ڼ��ɵ�·�����������Ʊ���Ӧԭ��Ϊ��Al2O3+N2+3C�T2AlN+3CO���Ʊ������У�ԭ����Ȳ�����Ӧ����ȫ�����ض�����ɲ�Ʒ�к���̼��Al2O3�����ʣ�Ϊ�ⶨ��Ʒ��AlN������������ijͬѧ����ͼ1װ�ý������ʵ�飮��ѡ�õĻ�ѧ�Լ�Ϊ��mg������Ƭ״���塢NaOH������Һ��ˮ��ú�͡�ҽ�þƾ����ش��������⣺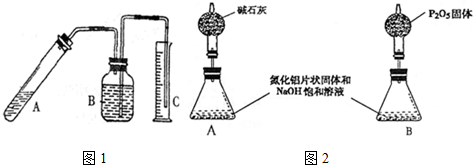
��1������������װ�������Եķ�������C�е��ܽ���ˮ�У����Թ�A��C�е��ܳ����������ݳ�����ȴ��C�е������и߳�Һ���ˮ�����½�����������B�м���ú�ͣ�Ȼ���Թ�A�����ˮ�У�B�е��������ݽ���B�����Թ�A�ָ����£�B�е����и߳�Һ����������½�����˵�����������ã�����װ��©����
��2����ͼB���Լ����ѡ�ã�����ţ����ڣ�
��ˮ��ú�͢�ҽ�þƾ�
��֪A ����NaAlO2���ɣ����ų��д̼�����ζ���壬д��A�з�����Ӧ�Ļ�ѧ����ʽAlN+NaOH+H2O=NaAlO2+NH3����
��3����ȡC����Ͳ����Һ�����ʱ��Ӧע��b��c��d������ţ���
a��C��Һ�治������ʱ�������� b�������ƶ�C��ʹ֮Һ����B��Һ����ƽ
c��A�в����������ݳ� d�������밼Һ����ʹ���ƽ
��4��mg������������NaOH������Һ��Ӧ��ʵ�������C���ռ���Һ������ΪVL��������ɱ�״��������AlN��Ʒ�Ĵ���Ϊ$\frac{41V}{22.4m}��100%$��д�������ʽ����
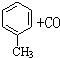
��5������ʵ�鷽��������������������������������ϴ����˽����������A��B����װ���е�һ�֣���ͨ����ڽ��У���ֻ����м��ֱ�Ҫ�����ݲ�д���ò������ͿɱȽ�ȷȷ����Ʒ��AlN�������������Ϻ�����װ����ͼ2�ǣ�A������ţ���
���� ��1��ֻҪ�Ƚ�װ���ܷ���������������ԭ��������������֤��
��2������ͼ1װ�ÿ�֪��A�е�����������������Һ��Ӧ����NaAlO2�����ų��д̼�����ζ����Ϊ������ʵ����Ҫ��ð����������ͨ������������ⶨ��Ʒ��AlN��������������������������ˮ���ݴ˴��⣻
��3��Ϊ��ȷ��ð���������������A�з�Ӧ��ȫ���ڶ���Ͳ���ʱ����ʹC��Һ����B��Һ����ƽ�������밼Һ����ʹ���ƽ���ݴ˴��⣻
��4�����ݰ����������ͨ����Ӧ����ʽ�ɼ������Ʒ�д�AlN�����������ݴ���=$\frac{AlN������}{��Ʒ������}$��100%���㣻
��5������ͼ��֪���������������������հ�����ˮ�����ȣ�����ͼ2B��װ�õ������ڷ�Ӧǰ��û�б仯��A�з�Ӧ�����İ����ӷ�������������������������װ�õ������仯�����Կ��Բ����ð��� �����������ݰ������������������������������������ȷ��AlN��Ʒ�Ĵ��ȣ��ݴ˴��⣮
��� �⣺��1������װ��ͼ��֪��������װ�������Եķ����ǽ�C�е��ܽ���ˮ�У����Թ�A��C�е��ܳ����������ݳ�����ȴ��C�е������и߳�Һ���ˮ�����½�����������B�м���ú�ͣ�Ȼ���Թ�A�����ˮ�У�B�е��������ݽ���B�����Թ�A�ָ����£�B�е����и߳�Һ����������½�����˵�����������ã�����װ��©����
�ʴ�Ϊ����C�е��ܽ���ˮ�У����Թ�A��C�е��ܳ����������ݳ�����ȴ��C�е������и߳�Һ���ˮ�����½�����������B�м���ú�ͣ�Ȼ���Թ�A�����ˮ�У�B�е��������ݽ���B�����Թ�A�ָ����£�B�е����и߳�Һ����������½�����˵�����������ã�����װ��©����
��2������ͼ1װ�ÿ�֪��A�е�����������������Һ��Ӧ����NaAlO2����Ӧ�ķ���ʽΪAlN+NaOH+H2O=NaAlO2+NH3�������ų��д̼�����ζ����Ϊ������ʵ����Ҫ��ð����������ͨ������������ⶨ��Ʒ��AlN��������������������������ˮ��ҽ�þƾ���Ҳ��ˮ������ѡ��ú�ͣ���ѡ �ڣ�
�ʴ�Ϊ���ڣ�AlN+NaOH+H2O=NaAlO2+NH3����
��3��Ϊ��ȷ��ð���������������A�з�Ӧ��ȫ���ڶ���Ͳ���ʱ����ʹC��Һ����B��Һ����ƽ�������밼Һ����ʹ���ƽ����ѡb��c��d��
��4�����ݷ�ӦAlN+NaOH+H2O=NaAlO2+NH3����֪�������������ΪVL�����ʵ���Ϊ$\frac{V}{22.4}$molʱ����μӷ�Ӧ��AlN������Ϊ$\frac{V}{22.4}$��41g������AlN��Ʒ����Ϊ$\frac{\frac{V}{22.4}��41}{m}��100%=\frac{41V}{22.4m}��100%$��
�ʴ�Ϊ��$\frac{41V}{22.4m}��100%$��
��5������ͼ��֪���������������������հ�����ˮ�����ȣ�����ͼ2B��װ�õ������ڷ�Ӧǰ��û�б仯��A�з�Ӧ�����İ����ӷ�������������������������װ�õ������仯�����Կ��Բ����ð��� �����������ݰ������������������������������������ȷ��AlN��Ʒ�Ĵ��ȣ�ͼ2��Aװ�ú�����
�ʴ�Ϊ��A��
���� ������ʵ������û�ѧ����ʽ�������ϵ���Ŀ����һ�����ۺ��ԣ�Ӧ�������������Ҫ����ס��������������ʵ���ⳣ�õķ�������Ŀ�Ѷ��еȣ�

| A�� | 150mL 1mol/L��AlCl3 | B�� | 75mL 2mol/L��Al��NO3��3 | ||
| C�� | 50mL 3mol/L��AlCl3 | D�� | 50mL 3mol/L��AlBr3 |
| A�� | ƫ��������Һ��ͨ�����CO2��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32- | |
| B�� | ��������������������Һ��Al2O3+2OH-=2AlO2-+H2O | |
| C�� | ʯ��ˮ�м�������С�մ�Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O | |
| D�� | ����ͭ��Һ������������Һ��ϣ�Cu2++2OH-=Cu��OH��2�� |
| A�� | ������ʯ���Ժ�ɫ | B�� | ʳ�û������ܷ���ˮ�ⷴӦ | ||
| C�� | ��װ�ò��Ͼ�����ϩ������ | D�� | PX��Ŀ�еĶԶ��ױ����ڱ����� |
| A�� | 2��3 | B�� | 1��2 | C�� | 1��3 | D�� | 3��2 |
��FeCl2
��Fe��OH��3
��Fe3O4
��FeCl3��
| A�� | ȫ�� | B�� | �ۢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
| A�� | ��������һ��û�зǼ��Լ� | |
| B�� | �����в����ܺ������Ӽ� | |
| C�� | ����Ԫ����ǽ���Ԫ�ؼ��γɵļ��������Ӽ� | |
| D�� | CO2��CH4������ÿ��ԭ���������γ���8���ӽṹ |
 $��_{����}^{AlCl_{3}��HCl}$
$��_{����}^{AlCl_{3}��HCl}$ 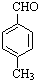 $��_{OH-����}^{CH_{3}CHO}$B$��_{��H+}^{��C}$
$��_{OH-����}^{CH_{3}CHO}$B$��_{��H+}^{��C}$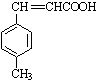 $��_{H_{2}SO_{4}������}^{CH_{3}OH}$E
$��_{H_{2}SO_{4}������}^{CH_{3}OH}$E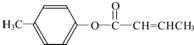 ��E��һ��ͬ���칹�壬������������NaOH��Һ���ȵĻ�ѧ����ʽΪ
��E��һ��ͬ���칹�壬������������NaOH��Һ���ȵĻ�ѧ����ʽΪ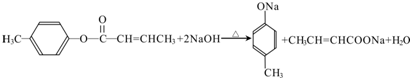 ��
��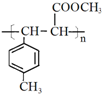 ��
��
