��Ŀ����
�л���A��̼��������Ԫ����ɣ���ȡ3��A��4.48L��������������ܱ�������ȼ�գ�ȼ�պ�����CO2��CO��H2O��g�������跴Ӧ��û��ʣ�ࣩ�������ɵ���������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������3.6�ˣ���ʯ������4.4�ˣ��ش��������⣺
��1����3��A�к�̼ԭ�����ʵ��� ����ԭ�����ʵ��� ��
��2���ܷ�ȷ�����л���A�ķ���ʽ��������д�����л������ʽ �������ܣ���˵������ ��
��3����A�м���Na�����ݲ�����д��A���ܵĽṹ��ʽ ��
��1����3��A�к�̼ԭ�����ʵ���
��2���ܷ�ȷ�����л���A�ķ���ʽ��������д�����л������ʽ
��3����A�м���Na�����ݲ�����д��A���ܵĽṹ��ʽ
���㣺�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
��������1��Ũ��������3.6gΪˮ����������ʯ������4.4gΪ������̼������������n=
���������̼��ˮ�����ʵ���������n=
�������������ʵ��������������غ����CO������������n=
����CO�����ʵ������ٸ���ԭ���غ���㣻
��2��������ԭ���غ�3gA����ԭ�ӵ��������ٸ��ݣ�1���еļ���ȷ�������ʵķ���ʽ��
��3��A�ķ���ʽΪC3H8O������1��Oԭ�ӣ��ܹ����Ʒ�Ӧ������������A�к����ǻ����ݴ�д�������ǻ���A�Ľṹ��ʽ��
| m |
| M |
| V |
| 22.4L/mol |
| m |
| M |
��2��������ԭ���غ�3gA����ԭ�ӵ��������ٸ��ݣ�1���еļ���ȷ�������ʵķ���ʽ��
��3��A�ķ���ʽΪC3H8O������1��Oԭ�ӣ��ܹ����Ʒ�Ӧ������������A�к����ǻ����ݴ�д�������ǻ���A�Ľṹ��ʽ��
���
�⣺��1��Ũ�������ص�Ϊˮ��������3.6gˮ�����ʵ���Ϊ��n��H2O��=
=0.2mol��
��ʯ�����ص�Ϊ������̼��������4.4g������̼�����ʵ���Ϊ��n��CO2��=
=0.1mol��
�����4.48L���������ʵ���Ϊ��
=0.2mol����������������0.2mol��32g/mol=6.4g
�����ɵ�CO������Ϊ��3g+6.4g-3.6g-4.4g=1.4g���� n��CO��=
=0.05mol��
��3gA�к��е���ԭ�ӵ����ʵ���Ϊ��n��H��=2n��H2O��=0.4mol��n��C��=n��CO2��+n��CO��=0.1mol+0.05mol=0.15mol��
�ʴ�Ϊ��0.15mol��0.4mol��
��2��3g A��n��H��=0.4mol��n��C��=0.15mol��
������ԭ���غ�ɵã�n��O��=2n��CO2��+n��CO��+n��H2O��-2n��O2��=2��0.1 mol+0.05 mol+0.2 mol-2��0.2 mol=0.05mol��
���ԣ�n��C����n��H����n��O��=3��8��1��
A�����ʽΪC3H8O����Hԭ����̼ԭ����Ŀ��֪����ԭ���Ѿ��ﵽ���ͣ���A�����ʽ����A�ķ���ʽ������A�ķ���ʽΪC3H8O��
�ʴ�Ϊ��C3H8O��
��3��A�м���Na�����ݲ�������˵��C3H8O�к��й������ǻ���-OH������AΪ������A���ܵĽṹ��ʽΪ��1-������CH3CH��OH��CH3��2-������CH3CH2CH2OH��
�ʴ�Ϊ��CH3CH��OH��CH3��CH3CH2CH2OH��
| 3.6g |
| 18g/mol |
��ʯ�����ص�Ϊ������̼��������4.4g������̼�����ʵ���Ϊ��n��CO2��=
| 4.4g |
| 44g/mol |
�����4.48L���������ʵ���Ϊ��
| 4.48L |
| 22.4/mol |
�����ɵ�CO������Ϊ��3g+6.4g-3.6g-4.4g=1.4g���� n��CO��=
| 1.4g |
| 28g/mol |
��3gA�к��е���ԭ�ӵ����ʵ���Ϊ��n��H��=2n��H2O��=0.4mol��n��C��=n��CO2��+n��CO��=0.1mol+0.05mol=0.15mol��
�ʴ�Ϊ��0.15mol��0.4mol��
��2��3g A��n��H��=0.4mol��n��C��=0.15mol��
������ԭ���غ�ɵã�n��O��=2n��CO2��+n��CO��+n��H2O��-2n��O2��=2��0.1 mol+0.05 mol+0.2 mol-2��0.2 mol=0.05mol��
���ԣ�n��C����n��H����n��O��=3��8��1��
A�����ʽΪC3H8O����Hԭ����̼ԭ����Ŀ��֪����ԭ���Ѿ��ﵽ���ͣ���A�����ʽ����A�ķ���ʽ������A�ķ���ʽΪC3H8O��
�ʴ�Ϊ��C3H8O��
��3��A�м���Na�����ݲ�������˵��C3H8O�к��й������ǻ���-OH������AΪ������A���ܵĽṹ��ʽΪ��1-������CH3CH��OH��CH3��2-������CH3CH2CH2OH��
�ʴ�Ϊ��CH3CH��OH��CH3��CH3CH2CH2OH��
���������⿼���л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ����������غ����һ����̼������Ϊ�����Ĺؼ���ע����ȷ�����غ���ȷ���л������ʽ���ṹ��ʽ�еķ�����

��ϰ��ϵ�д�
�����Ŀ
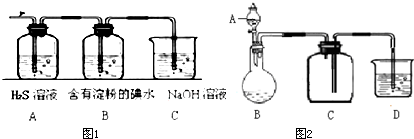
 ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
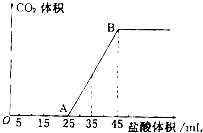
ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
 ��������10mol/L��ŨH2SO4���Ƴ�Ũ��Ϊ0.1mol/L�� 500mLϡH2SO4�IJ������밴Ҫ����գ�
��������10mol/L��ŨH2SO4���Ƴ�Ũ��Ϊ0.1mol/L�� 500mLϡH2SO4�IJ������밴Ҫ����գ� ����β���к���NO��CO���к����壮ѡ���ʵ��Ĵ����ɽ�CO��NOת��Ϊ�����壮
����β���к���NO��CO���к����壮ѡ���ʵ��Ĵ����ɽ�CO��NOת��Ϊ�����壮