��Ŀ����
14�� Ԫ�����ڱ������кͷ�����Ԫ�ػ�̬ԭ�Ӻ�����ӵ��Ų�ϢϢ��أ���ݴ˻ش��������⣺
Ԫ�����ڱ������кͷ�����Ԫ�ػ�̬ԭ�Ӻ�����ӵ��Ų�ϢϢ��أ���ݴ˻ش��������⣺��1������ԭ�ӽṹ��Ԫ�����ڱ��Ĺ�ϵ����������0���ԭ������Ϊ8��
��2��X��Y��Ϊ��2����p��Ԫ�أ���ԭ�ӵĵ�һ��������ֵ���Ӵ�С��˳����ͬ����Ԫ���д��ڵ���λ�͵���λ��
�پ���Y�Ľṹ��Ԫ����Yԭ����ɵ�����ʮ���壨��ͼ��ʾ����������20���ȱ������ε����һ����Ŀ�Ķ��㣬ÿ���������һ��Yԭ�ӣ��ʴ˻����ṹ��Ԫ����12��Yԭ�ӹ��ɣ�������30��Y-Y����
������һ������ʮ�����е�ÿһ��������ȥ���ɻ��ֻ���������κ�������ι��ɵĶ����壮���������ÿһ�������Ϊ̼ԭ�ӣ��ɵõ�һ��C60���ӵĽṹģ�ͣ���1molC60�����к���a������ĿΪ90NA��C60�����ȡ�����������ܶѻ���������λ��Ϊ12��
��X��Y���γɶ������͵Ľṹ������˵����ȷ����ab��
a����������ʯī��YX�������������
b������������ʯ��YX�����dz�Ӳ���ϣ����������ĥ��
c������������ʯ��YX�����У�Yԭ�ӵ��ӻ��������Ϊsp2
��3��ZΪ��4����d��Ԫ�أ����Ϊ+7�ۣ�M��Z��ͬ���ڵ�Ԫ�أ�������ͬ������ϼۣ�
��д��Z�Ļ�̬ԭ�Ӽ۲�����Ų�ʽ3d54s2��
������ǿ����HMO��HMO2��ԭ����HMO2�з��ǻ���ԭ����Ŀ��HMO�Ķ࣮
��ZԪ�ؿɲ����γ����壮������һ����ƽ�����������ϸ�����λ����Ķ��ؾ��壬��ͨ��X-��������ʵ�����־��塢����ͷǾ��壮
�ܾ���Z���γ����������������������о��������Zԭ�Ӻ˼����Ϊapm����Z������ܶ�Ϊ$\frac{165\sqrt{3}��1{0}^{30}}{4{a}^{3}{N}_{A}}$g•cm-3��
���� ��1�����ݸ���������Ԫ���������㣻
��2��ͬ������ϡ�������һ�������������Ԫ����ԭ���������Ԫ�ص�һ�����ܳ��������ƣ�����A�塢��A������ܼ��ֱ�Ϊȫ���������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ��ʵڶ������е�һ������˳��Ϊ��Ne��F��N��O��C��Be��B��Li��X��Y��Ϊ��2����p��Ԫ�أ���ԭ�ӵĵ�һ��������ֵ���Ӵ�С��˳����ͬ����Ԫ���д��ڵ���λ�͵���λ����XΪNԪ�ء�YΪBԪ�أ�
��ÿ�������κ���3��Yԭ�ӣ�ÿ��Yԭ��Ϊ5�������ι��ã�ÿ�������κ���3��Y-Y������ÿ��Y-Y��Ϊ2�������ι��ã����þ�̯�����㣻
������һ������ʮ�����е�ÿһ��������ȥ���ɻ��ֻ���������κ�������ι��ɵĶ����壮���������ÿһ�������Ϊ̼ԭ�ӣ���ÿ��̼ԭ���γ�3��̼̼�Ҽ���ÿ��̼̼�Ҽ�Ϊ1��̼ԭ���ṩ$\frac{1}{2}$�����þ�̯������1molC60�����к��ЦҼ�����Ŀ��
C60�����ȡ�����������ܶѻ����Զ���C60�о�����֮���ڵ�C60�������ģ�ÿ������Ϊ8���������ã���ÿ����Ϊ2���������ã�
��a����������ʯī��YX���壬���Ϊ���Ӽ���������������С��
b������������ʯ��YX���壬Ϊ�ռ���״�ṹ������ԭ�Ӿ��壻
c������������ʯ��BN�����У�Bԭ���γ�4��B-N��������1����λ�������ӻ������ĿΪ4��
��3��ZΪ��4����d��Ԫ�أ����Ϊ+7�ۣ���ZΪMnԪ�أ�M��Z��ͬ���ڵ�Ԫ�أ�������ͬ������ϼۣ���MΪBr��
��Z���ڵĵ������ڵڢ�B�壻
�ڷ��ǻ�����ĿԽ�࣬����Խǿ��
��ͨ��X-��������ʵ�����־��塢����ͷǾ��壮
�ܾ���Z���γ�����������������Խ�����3��Zԭ�����ڣ��������о��������Zԭ�Ӻ˼����Ϊapm������Խ��߳���Ϊ2a pm���ʾ����ⳤΪ$\frac{2a}{\sqrt{3}}$pm�����ݾ�̯�����㾧����Zԭ����Ŀ����ʾ�������������ٸ��ݦ�=$\frac{m}{V}$����Z������ܶȣ�
��� �⣺��1��He��ԭ������Ϊ2�����ݸ���������Ԫ����������֪��������0���ԭ������Ϊ2+8+8+18+18+32+32=118��
�ʴ�Ϊ��118��
��2��ͬ������ϡ�������һ�������������Ԫ����ԭ���������Ԫ�ص�һ�����ܳ��������ƣ�����A�塢��A������ܼ��ֱ�Ϊȫ���������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ��ʵڶ������е�һ������˳��Ϊ��Ne��F��N��O��C��Be��B��Li��X��Y��Ϊ��2����p��Ԫ�أ���ԭ�ӵĵ�һ��������ֵ���Ӵ�С��˳����ͬ����Ԫ���д��ڵ���λ�͵���λ����XΪNԪ�ء�YΪBԪ�أ���
��ÿ�������κ���3��Yԭ�ӣ�ÿ��Yԭ��Ϊ5�������ι��ã�����Yԭ����ĿΪ$\frac{20��3}{5}$=12��
ÿ�������κ���3��Y-Y������ÿ��Y-Y��Ϊ2�������ι��ã�����Y-Y����ĿΪ$\frac{20��3}{2}$=30��
�ʴ�Ϊ��12��30��
������һ������ʮ�����е�ÿһ��������ȥ���ɻ��ֻ���������κ�������ι��ɵĶ����壮���������ÿһ�������Ϊ̼ԭ�ӣ�ÿ��̼ԭ��Ϊ1������Ρ�2�������ι��ã���ÿ��̼ԭ���γ�3��̼̼�Ҽ���ÿ��̼̼�Ҽ�Ϊ1��̼ԭ���ṩ$\frac{1}{2}$����1molC60�����к��ЦҼ�Ϊ$\frac{60��3}{2}$mol=90mol��������90NA ���Ҽ���
��C60�����ȡ�����������ܶѻ����Զ���C60�о�����֮���ڵ�C60�������ģ�ÿ������Ϊ8���������ã���ÿ����Ϊ2���������ã�������λ��Ϊ$\frac{3��8}{2}$=12��
�ʴ�Ϊ��90NA��12��
��a����������ʯī��YX���壬���Ϊ���Ӽ���������������С���ʵ�������������������a��ȷ��
b������������ʯ��YX���壬Ϊ�ռ���״�ṹ������ԭ�Ӿ��壬��Ӳ���ϣ����������ĥ�ԣ���b��ȷ��
c������������ʯ��BN�����У�Bԭ���γ�4��B-N��������1����λ�������ӻ������ĿΪ4��Bԭ�Ӳ�ȡsp3�ӻ�����c����
�ʴ�Ϊ��ab��
��3��ZΪ��4����d��Ԫ�أ����Ϊ+7�ۣ���ZΪMnԪ�أ�M��Z��ͬ���ڵ�Ԫ�أ�������ͬ������ϼۣ���MΪBr��
��Z���ڵĵ������ڵڢ�B�壬�۵����Ų�ʽΪ3d54s2���ʴ�Ϊ��3d54s2��
��HMO2�з��ǻ���ԭ����Ŀ��HMO�Ķ࣬������HMO��HMO2���ʴ�Ϊ������HMO2�з��ǻ���ԭ����Ŀ��HMO�Ķࣻ
��ͨ��X-��������ʵ�����־��塢����ͷǾ��壬�ʴ�Ϊ��X-��������ʵ�飻
�ܾ���Z���γ�����������������Խ�����3��Zԭ�����ڣ��������о��������Zԭ�Ӻ˼����Ϊa pm������Խ��߳���Ϊ2a pm���ʾ����ⳤΪ$\frac{2a}{\sqrt{3}}$pm��������Mnԭ����ĿΪ8��$\frac{1}{8}$+1=2��������Ϊ2��$\frac{55}{{N}_{A}}$g���ʾ�����ܶ�Ϊ2��$\frac{55}{{N}_{A}}$g�£�$\frac{2a}{\sqrt{3}}$��10-10 cm��3=$\frac{165\sqrt{3}��1{0}^{30}}{4{a}^{3}{N}_{A}}$g•cm-3��
�ʴ�Ϊ��$\frac{165\sqrt{3}��1{0}^{30}}{4{a}^{3}{N}_{A}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰Ԫ�����ڱ��������ܡ���������Ų����ӻ���ʽ���������������ʡ���������ȣ����ؾ�������Ŀ��飬��Ҫѧ���߱�һ���Ŀռ���������ѧ�����������ѶȽϴ������״���Ŀ��

| A�� | ${\;}_{1}^{1}$H��${\;}_{1}^{3}$H | B�� | ������춡�� | C�� | O2��O3 | D�� | H2O��H2O2 |
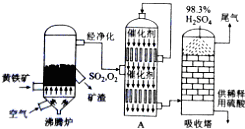
��1�����᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO2�ڷ���¯�л��϶��ɣ���CuFeS2�ǻ��������һ�ɷ֣�д������CuFeS2�Ļ�ѧ����ʽΪ4CuFeS2+13O2$\frac{\underline{\;����\;}}{\;}$ 4CuO+2Fe2O3+8SO2��
��2������ͼ���豸A�ǽӴ��ң����豸���ƣ������и��豸�ϲ�Ѹ��ʹ�õ�����ΪSO2��O2��
��3������������98.3%Ũ��������SO2��ԭ���ǿ��Է�ֹ�γ�������ʹ��������������ȫ��
��4�����ݹ�������ͼ�������Ƕ��ж�����˵����ȷ����ABDE������ţ�
A��Ϊʹ��������ȼ�գ��轫�����
B������¯���ų���¯���ɹ�����
C��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
D��β����SO2����NaOHŨ��Һ������
E�����᳧�ij�ַ��ѡ��������������Ĺ�ҵ���еĽ���
��5������¯�ų���¯���е�����ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
| ����¯�¶�/�� | 600 | 620 | 640 | 660 |
| ¯����CuSO4����������/% | 9.3 | 9.2 | 9.0 | 8.4 |
��6��ij���᳧Ϊ�ⶨ�豸A������������SO2�����������ȡ280mL��������ɱ�״����������Ʒ������Fe��SO4��3��Һ��ȫ��Ӧ������������Ӱ�죩����Ũ��Ϊ0.02mol/L��K2Cr2O7����Һ�ζ����е㣬����K2Cr2O7��Һ25.00mL����Ӵ�������������SO2���������Ϊ12.00%��
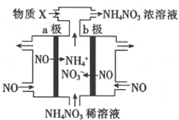 ���NO�Ʊ�NH4NO3�Ĺ���ԭ����ͼ��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3��ͨ������X������˵������ȷ���ǣ�������
���NO�Ʊ�NH4NO3�Ĺ���ԭ����ͼ��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3��ͨ������X������˵������ȷ���ǣ�������| A�� | a���ӵ�Դ�ĸ��� | |
| B�� | ������ӦΪ��NO-3e-+2H2O=NO3-+4H+ | |
| C�� | ������ӦΪ��NO+5e-+6H+=NH4++H2O | |
| D�� | XΪNH3�����Ƶ�3molNH4NO3�������貹��2molX���� |
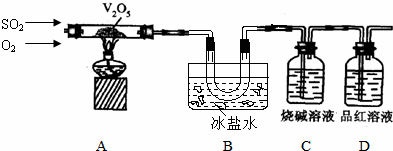
| A�� | ������������Y��Z | |
| B�� | �˵������a��b | |
| C�� | �ȶ��ԣ�H2Y��HZ | |
| D�� | X����һ���ܴ�W������Һ���û���W���� |
 ��
��
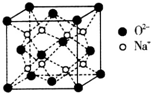 H��C��N��O��Na��Fe��Cu�dz���������Ԫ�أ���ش��������⣺
H��C��N��O��Na��Fe��Cu�dz���������Ԫ�أ���ش��������⣺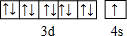 ��
��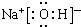 ��
��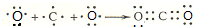
 ��
��