��Ŀ����
6��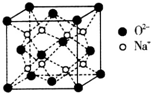 H��C��N��O��Na��Fe��Cu�dz���������Ԫ�أ���ش��������⣺
H��C��N��O��Na��Fe��Cu�dz���������Ԫ�أ���ش��������⣺��1��N��O��Naԭ�ӵ�һ��������С�����˳����Na��O��N����Ԫ�ط��ź͡�������ʾ����Cuԭ�ӵ��������ӹ��ʽΪ
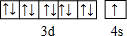 ��
����2��N��Na+��Fe3+��Cu2+��������̬�ĺ�������Ų�ʽ��δ�ɶԵ�����������Fe3+��Cu2+��ˮ�г�����ɫ����Ϊ�γ���ˮ��ͭ���ӣ��仯ѧʽΪ[Cu��H2O��4]2+��ˮ������ͭ���Ӽ��ϵĻ�ѧ������Ϊ��λ����
��3�����ݼ۲���ӶԻ��������ж����з��ӻ������пռ乹����V�ε��Ǣڢܣ�����ţ�����H3O+����H2O����NO2+����NO2-��
��4�����ӣ�CN��2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ������������8�����ȶ��ṹ����ṹʽΪN��C-C��N��1�������к���4���м���
��CN��2��Ϊ����±�ء�����������Cl2�Ļ�ѧ���ʣ���CN��2��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ��CN��2+2NaOH=NaCN+NaCNO+H2O��
��5��O��Na�γɵ�һ��ֻ�������Ӽ������ӻ�����侧���ṹ��ͼ�������ӻ�����Ļ�ѧʽΪNa2O����֪�þ������ܶ�Ϊ��g/cm3�������ӵ�����ΪNA�����߳�a=$\root{3}{\frac{248}{��{N}_{A}}}$cm�����ú��ѡ�NA�Ĵ���ʽ��ʾ����
���� ��1���ǽ�����Խǿ����һ������Խ��Ԫ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ�����Ԫ�صģ�
Cuԭ��Cuԭ�ӵ����������Ų�ʽΪ3d104s1����������ԭ�������ع���������ʽ��
��2��Fe3+����Χ�����Ų�Ϊ3d5��δ�ɶԵ�������ࣻCu2+��ˮ�г�����ɫ����Ϊ�γ���ˮ��ͭ���ӣ���λ��Ϊ4��ˮ������ͭ���Ӽ�ͨ����λ����ϣ�
��3����������ԭ�ӹµ��Ӷ������۲���Ӷ������ж����ռ�ṹ������ԭ�ӹµ��Ӷ���=$\frac{1}{2}$��a-bx����aΪ����ԭ�Ӽ۵�������xΪ����ԭ�ӽ�ϵ�ԭ����Ŀ��bΪ������ԭ�ӽ�ϵ�ԭ������ܽ��ܵĵ��������۲���Ӷ���=�Ҽ����Ӷ���+�µ��Ӷ������Ҽ����Ӷ�����������ԭ�ӽ�ϵ�ԭ����Ŀ��
��4�����ӣ�CN��2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ������������8�����ȶ��ṹ��Cԭ���γ�4�����ۼ���Nԭ���γ�3�����ۼ�������ṹ��ʽΪN��C-C��N��
�����������������Ƶķ�Ӧ��д����ʽ��
��5�����ݾ�̯�����㾧����Na+��O2+������Ŀ������ȷ����ѧʽ����ʾ�������������ٽ��m=��V=��a3���㾧���ⳤ��
��� �⣺��1���ǽ�����Խǿ����һ������Խ��Ԫ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ�����Ԫ�صģ��ʵ�һ������Na��O��N��
Cuԭ��Cuԭ�ӵ����������Ų�ʽΪ3d104s1������ʽΪ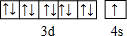 ��
��
�ʴ�Ϊ��Na��O��N��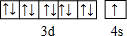 ��
��
��2��N��Χ�����Ų�Ϊ2s22p3��ԭ����3��δ�ɶԵ��ӣ�Na+���������Ų�Ϊ2s22p6��û�е����ӣ�Fe3+����Χ�����Ų�Ϊ3d5����5�������ӣ�Cu2+����Χ�����Ų�Ϊ3d9����1�����ӣ�
Cu2+��ˮ�г�����ɫ����Ϊ�γ���ˮ��ͭ���ӣ��仯ѧʽΪ[Cu��H2O��4]2+��ˮ������ͭ���Ӽ��ϵĻ�ѧ��Ϊ��λ����
�ʴ�Ϊ��Fe3+��[Cu��H2O��4]2+����λ����
��3����H3O+��Oԭ�ӹµ��Ӷ���=$\frac{6-1-1��3}{2}$=1���۲���Ӷ���=3+1=4���ʿռ乹��Ϊ�����Σ�
��H2O��Oԭ�ӹµ��Ӷ���$\frac{6-1��2}{2}$=2���۲���Ӷ���=2+2=4���ʿռ乹��ΪV�Σ�
��NO2+��Nԭ�ӹµ��Ӷ���=$\frac{5-1-2��2}{2}$=0���۲���Ӷ���=2+0=2���ʿռ乹��Ϊֱ���Σ�
��NO2-��Nԭ�ӹµ��Ӷ���=$\frac{5+1-2��2}{2}$=1���۲���Ӷ���=2+1=3���ʿռ乹��ΪV�Σ�
�ʴ�Ϊ���ڢܣ�
��4�����ӣ�CN��2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ������������8�����ȶ��ṹ��Cԭ���γ�4�����ۼ���Nԭ���γ�3�����ۼ�������ṹ��ʽΪN��C-C��N��1�������к���4���м���
��CN��2��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ����CN��2+2NaOH=NaCN+NaCNO+H2O��
�ʴ�Ϊ��N��C-C��N��4����CN��2+2NaOH=NaCN+NaCNO+H2O��
��5��Na+�ھ����ڲ�������8����������O2-��ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��������Ŀ֮��Ϊ2��1���ʻ�ѧʽΪNa2O����������Ϊ4��$\frac{62}{{N}_{A}}$���������ܶ�Ϊ��g/cm3����4��$\frac{62}{{N}_{A}}$=��g/cm3����a cm��3�����a=$\root{3}{\frac{248}{��{N}_{A}}}$��
�ʴ�Ϊ��Na2O��$\root{3}{\frac{248}{��{N}_{A}}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ������۲���Ӷ����������ۡ����ӽṹ����������ȣ���Ҫѧ���߱���ʵ�Ļ�����ע��ͬ���ڵ�һ�������쳣�����

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�| A�� | Ԫ��Y��Z��W�γɵ����Ӿ�����ͬ���Ӳ�ṹ�����Ӱ뾶�������� | |
| B�� | 39g Z2Y2�к��е�������ԼΪ1.204��1024 | |
| C�� | Y���������Ϊ+6 | |
| D�� | Ԫ��Y��R�ֱ���Ԫ��X�γɵĻ���������ȶ��ԣ�XmY��XmR |
| A�� | ���������ɫ���ٱ仯 | B�� | ��������ܶȲ��ٱ仯 | ||
| C�� | 2v����N2O4��=v�棨NO2�� | D�� | ���������Է����������� |
 �����£���0.1000mol/L����ζ�25mL0.1000mol/LNa2A��Һ����֪H2AΪ��Ԫ���ᣩ����ζ�������ͼ��ʾ�����жԵζ���������Һ���������Ũ�ȹ�ϵ�ж���ȷ���ǣ�������
�����£���0.1000mol/L����ζ�25mL0.1000mol/LNa2A��Һ����֪H2AΪ��Ԫ���ᣩ����ζ�������ͼ��ʾ�����жԵζ���������Һ���������Ũ�ȹ�ϵ�ж���ȷ���ǣ�������| A�� | a�㣺c��A2-��=c��HA-�� | B�� | b�㣺5c��Cl-��=4[c��A2-��+c��HA-��+c��H2A��] | ||
| C�� | c�㣺c��Na+����c��HA-����c��A2-����c��H2A�� | D�� | d�㣺c��H+��=c��HA-��+c��A2-��+c��OH-�� |
| A�� | ��ά��ˮ��IJ���������Ҵ� | |
| B�� | ��ϩ������ϩ��������ϩ����������������ʹ��ˮ��ɫ | |
| C�� | ����-20���ɻ���ʹ�õ�̼��ά��һ�����͵��л��߷��Ӳ��� | |
| D�� | NH4NO3��KNO3��KClO3��Na2S��C2H5OH��ϴ�����ͬһ�ֿ� |
| A�� | CH3-CH2-NO2 �� H2N-CH2-COOH | B�� | H��D | ||
| C�� | ����������� | D�� | ���Ͱ��� |
��1��AԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ������Ԫ�����ڱ��е�λ��Ϊ�������ڵ�VIIA��
��2��BԪ�ػ�̬ԭ�ӵ��������2��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ������Ϊ̼����
��3��DԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�D�����������Ų�ʽ
Ϊ4s1
��4��XԪ�ص�ԭ�����������Ų�ʽΪnsnnpn+1��YԪ�ص�ijͬλ��ԭ�������ӣ�X��Y�γɵ��������ĽṹʽΪ

��5��MԪ�ص����������ӵ�3d�ܼ�Ϊ�������M��̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2
��6�������N��ʧȥ���ӵĵ����ܣ�
| I1 | I2 | I3 | I4 | I5 | I6 | |
| In | 578 | 1817 | 2745 | 11578 | 14831 | 18378 |
 Ԫ�����ڱ������кͷ�����Ԫ�ػ�̬ԭ�Ӻ�����ӵ��Ų�ϢϢ��أ���ݴ˻ش��������⣺
Ԫ�����ڱ������кͷ�����Ԫ�ػ�̬ԭ�Ӻ�����ӵ��Ų�ϢϢ��أ���ݴ˻ش��������⣺