��Ŀ����
16��25��ʱ����1LŨ�Ⱦ�Ϊ1mol•L-1�����ᣨHA�������Σ�NaA����ɵĻ����Һ��ͨ��HCl��������NaOH����ʱ����ҺpH�����n��H+����n��OH-�����仯��������ͼ�������й�˵��������ǣ�������
| A�� | b����Һ��c��A-����c��Na+����c��HA�� | |
| B�� | ͨ��HCl��c��HA��/c��A-������ | |
| C�� | ������1 mol NaOH����Һ��c��Na+��=c��A-�� | |
| D�� | a��b��c������Һ��ˮ�ĵ���̶��������� |
���� A��δ��HCl��NaOHʱ����Һ�����ԣ�˵��HA�ĵ���̶ȴ���A-ˮ��̶ȣ�
B��ͨ��HCl��NaA��HCl��Ӧ����HA��
C������1mol NaOH��pH��7����Һ�ʼ��ԣ���ϵ���غ��жϣ�
D��A��B��Һ�����ԣ�����ˮ�ĵ��룬C�����ԣ��Դ��жϣ�
��� �⣺A��δ��HCl��NaOHʱ����Һ�����ԣ�˵��HA�ĵ���̶ȴ���A-ˮ��̶ȣ���b����Һ��c��A-����c��Na+����c��HA������A��ȷ��
B��ͨ��HCl��NaA��HCl��Ӧ����HA������c��HA������c��A-����С������c��HA��/c��A-������B��ȷ��
C������1mol NaOH��pH��7����Һ�ʼ��ԣ�����H+����c��OH-�����ɵ���غ��֪c��Na+����c��A-������C����
D��A��B��Һ�����ԣ�����ˮ�ĵ��룬C�����ԣ�pHԽС������Խǿ��ˮ�ĵ���̶�ԽС����a��b��c��������ʾ����Һ��ˮ�ĵ���̶���������D��ȷ��
��ѡC��
���� ���⿼������ϵĶ����жϺͼ��㣬������ѧ���ķ��������ͼ��������Ŀ��飬ע�����ͼ��������ϢΪ������Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
6������������ȷ���ǣ�������
| A�� | H2��Cl2�о���ȼ�գ�������ɫ���棬ð���� | |
| B�� | SiO2�Ļ�ѧ���ʲ����ã������κ��ᷢ����Ӧ | |
| C�� | �����£���������Ũ����ᷢ���ۻ���Ӧ | |
| D�� | ������ͨ����ɫʯ����Һ��һЩʱ�����Һ��� |
5��ͭ���仯�����ڹ�ũҵ�������ճ�������Ӧ�÷dz��㷺��ij�о�С���ô�ͭ�������������Ʊ��Ȼ�ͭ���壨CuCl2•2H2O �����������£�

��֪�����£�Cu2+��Fe3+���������↑ʼ�����ͳ�����ȫʱ��pH���±���
��ش��������⣺
��1����ҺI�м����Լ�X���Ե�����ҺpH���Ӷ���ȥFe3+�Ҳ��������ʣ�
���Լ�X��ѡ�����������е�ad������ţ���
a��CuO b��NaOH c��Cu d��Cu��OH��2
�ڵ�����ҺpHʱ�������Ͽ�ѡ��pH���Χ��3.2��pH��4.7��
��2������Һ���Ʊ�CuCl2•2H2O�IJ�������Ϊ���ߵμ�Ũ�������Ũ������ȴ�ᾧ����������ƣ������ˡ�ϴ�Ӹ��
��3�������£���NaOH��Һ��μ��뵽Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��CuCl2�����Һ�У������ɵij�����Cu��OH��2��������ʱKSP[Mg��OH��2]=1.8��10-11��KSP[Cu��OH��2]=2.2��10-20��
��4��ijѧϰС���õ������ⶨCuCl2•2H2O��Ʒ�Ĵ��ȣ����ʲ��뷢����Ӧ����ʵ�����£�
a��ȷ��ȡCuCl2•2H2O��Ʒmg��С�ձ��У�������������ˮ�������ĵ⻯�أ��ٵ���������ϡ���ᣬ��ַ�Ӧ�����û��Һ���250mL������Һ������֪��2Cu2++4I-�T2CuI+I2��
b����ȡ25.00mL������Һ����ƿ�У��Ӽ���ָʾ������c mol•L-1Na2S2O3��Һ�ζ����յ㣬�ظ�2�Σ�������ı�Һ�����ƽ��ֵΪV mL������֪��I2+2S2O32-�T2I-+S4O62-��
��ʵ����ʹ�õ�ָʾ������Ϊ���ۣ��ﵽ�ζ��յ�ʱ����Һ��ɫ�仯Ϊ��Һ����ɫ��Ϊ��ɫ��
�ڸ���Ʒ��CuCl2•2H2O ����������Ϊ$\frac{{171cV��{{10}^{-3}}��10}}{m}��100%$���ú�m��c��V�Ĵ���ʽ��ʾ�����û���

��֪�����£�Cu2+��Fe3+���������↑ʼ�����ͳ�����ȫʱ��pH���±���
| �������� | Fe3+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 6.7 |
��1����ҺI�м����Լ�X���Ե�����ҺpH���Ӷ���ȥFe3+�Ҳ��������ʣ�
���Լ�X��ѡ�����������е�ad������ţ���
a��CuO b��NaOH c��Cu d��Cu��OH��2
�ڵ�����ҺpHʱ�������Ͽ�ѡ��pH���Χ��3.2��pH��4.7��
��2������Һ���Ʊ�CuCl2•2H2O�IJ�������Ϊ���ߵμ�Ũ�������Ũ������ȴ�ᾧ����������ƣ������ˡ�ϴ�Ӹ��
��3�������£���NaOH��Һ��μ��뵽Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��CuCl2�����Һ�У������ɵij�����Cu��OH��2��������ʱKSP[Mg��OH��2]=1.8��10-11��KSP[Cu��OH��2]=2.2��10-20��
��4��ijѧϰС���õ������ⶨCuCl2•2H2O��Ʒ�Ĵ��ȣ����ʲ��뷢����Ӧ����ʵ�����£�
a��ȷ��ȡCuCl2•2H2O��Ʒmg��С�ձ��У�������������ˮ�������ĵ⻯�أ��ٵ���������ϡ���ᣬ��ַ�Ӧ�����û��Һ���250mL������Һ������֪��2Cu2++4I-�T2CuI+I2��
b����ȡ25.00mL������Һ����ƿ�У��Ӽ���ָʾ������c mol•L-1Na2S2O3��Һ�ζ����յ㣬�ظ�2�Σ�������ı�Һ�����ƽ��ֵΪV mL������֪��I2+2S2O32-�T2I-+S4O62-��
��ʵ����ʹ�õ�ָʾ������Ϊ���ۣ��ﵽ�ζ��յ�ʱ����Һ��ɫ�仯Ϊ��Һ����ɫ��Ϊ��ɫ��
�ڸ���Ʒ��CuCl2•2H2O ����������Ϊ$\frac{{171cV��{{10}^{-3}}��10}}{m}��100%$���ú�m��c��V�Ĵ���ʽ��ʾ�����û���
6��25��ʱ��ij������Һ��ֻ��NH4+��Cl-��H+��OH-�������ӣ�����˵������ȷ���ǣ�������
| A�� | ������pH=2��������pH=12�İ�ˮ�������϶��� | |
| B�� | ����Һ�����ɵ����ʵ���Ũ�ȵ�����Ͱ�ˮ�������϶��� | |
| C�� | ����������ˮ����Һ������Ũ�ȿ���Ϊ��c ��NH4+����c ��Cl-����c ��OH-����c��H+�� | |
| D�� | ����Һ��c ��NH4+��=c ��Cl-��+c ��OH-��-c��H+�� |


 ��
�� HClO+OH-��
HClO+OH-��
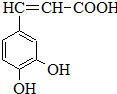 �����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�
�����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�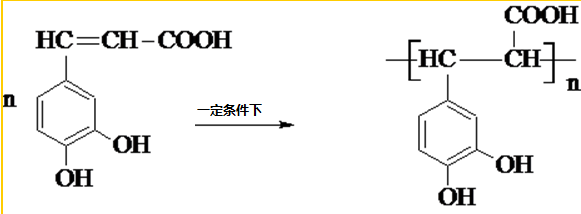 ��
��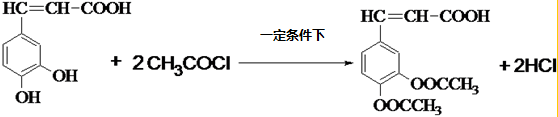 ��
��