��Ŀ����
С����Ҫ����80g��������Ϊ10%������������Һ��������Ҷ����ǩ������ͼ�����Ƹ�����������Һ��ʵ�����ʾ��ͼ��
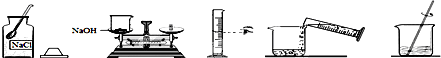
��ʵ�鲽�衿
��1�����㣺��Ҫ�������ƹ��� g��ˮ mL��ˮ���ܶ���1.0g/cm3�ƣ�
��2����������������ƽ��ȡ�������ƹ��壬�ù��Ϊ ���10mL������50mL����100mL��������Ͳȡ����Ҫ��ˮ������ʢ���������Ƶ��ձ��У�
��3���ܽ⣺�ò��������裬ʹ�������ƹ�����ȫ�ܽ⣮
����չ˼ά��
����С�������������Ƶ�����������Һ��ȫ�к�������������Ϊ10%�����ᣬ������������������Ƕ��٣�
����Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl=NaCl+H2O��

��ʵ�鲽�衿
��1�����㣺��Ҫ�������ƹ���
��2����������������ƽ��ȡ�������ƹ��壬�ù��Ϊ
��3���ܽ⣺�ò��������裬ʹ�������ƹ�����ȫ�ܽ⣮
����չ˼ά��
����С�������������Ƶ�����������Һ��ȫ�к�������������Ϊ10%�����ᣬ������������������Ƕ��٣�
����Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl=NaCl+H2O��
���㣺��Һ������
ר�⣺ʵ����
��������ʵ�鲽�衿
��1��������������=��Һ���������ʵ������������ɸ�����Һ�����������ʵ�������������������Һ����Ҫ�����ʵ��������ٸ����ܼ�����=��Һ����-���������������ˮ��������
��2����������ȡҺ��������ȷ����ѡ��Ͳ�Ĺ��
����չ˼ά�����û�ѧ����ʽ����������
��1��������������=��Һ���������ʵ������������ɸ�����Һ�����������ʵ�������������������Һ����Ҫ�����ʵ��������ٸ����ܼ�����=��Һ����-���������������ˮ��������
��2����������ȡҺ��������ȷ����ѡ��Ͳ�Ĺ��
����չ˼ά�����û�ѧ����ʽ����������
���
�⣺��ʵ�鲽�衿
��1����������=��Һ���������ʵ���������������100g������������Ϊ5%���Ȼ�����Һ�����Ȼ��Ƶ�����=80g��10%=8g���ܼ�����=��Һ����-����������������ˮ������=80g-8g=72g��ˮ�����Ϊ��72mL���ʴ�Ϊ��8��72��
��2����Ϊ����Ҫ��ˮ�������72mL����������ȡҺ��������ȷ����ѡ��Ͳ�Ĺ������ѡ�ù��Ϊ100mL����Ͳ��ȡ����Ҫ��ˮ���ʴ�Ϊ��100mL��
����չ˼ά��
������������������Ϊx
NaOH+HCl�TNaCl+H2O
40 36.5
8g x
=
x=7.3g
������������=
=73g
�𣺹�����������������Ϊ10%������73g��
��1����������=��Һ���������ʵ���������������100g������������Ϊ5%���Ȼ�����Һ�����Ȼ��Ƶ�����=80g��10%=8g���ܼ�����=��Һ����-����������������ˮ������=80g-8g=72g��ˮ�����Ϊ��72mL���ʴ�Ϊ��8��72��
��2����Ϊ����Ҫ��ˮ�������72mL����������ȡҺ��������ȷ����ѡ��Ͳ�Ĺ������ѡ�ù��Ϊ100mL����Ͳ��ȡ����Ҫ��ˮ���ʴ�Ϊ��100mL��
����չ˼ά��
������������������Ϊx
NaOH+HCl�TNaCl+H2O
40 36.5
8g x
| 40 |
| 36.5 |
| 8g |
| X |
x=7.3g
������������=
| 7.3g |
| 10% |
�𣺹�����������������Ϊ10%������73g��
������ͨ���ش���֪�������ʡ��ܼ������ļ��㷽��������ƽ����ʱ��ע���������������������������һ������Һ�Ļ������裮

��ϰ��ϵ�д�
�����Ŀ
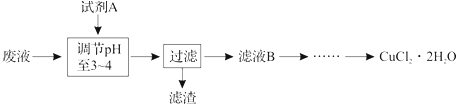
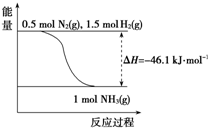
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ�������
A��MnO2������Ũ���ᷴӦ��ȡCl2��MnO2+4HCl
| ||||
| B��AlCl3��Һ�еμ�Ũ��ˮ��������Al3++4NH3?H2O�TAlO2-+4NH4++2H2O | ||||
| C���Ȼ�������Һ�м������3Fe2++4H++NO3-�T3Fe3++2H2O+NO�� | ||||
| D��NH4HCO3���ڹ�����NaOH��Һ�У�HCO3-+OH-�TCO32-+H2O |

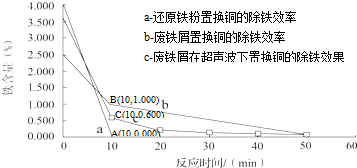
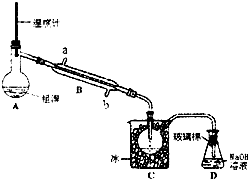 �屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ590C������ˮ���ж��Ժ�ǿ���ԣ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��
�屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ590C������ˮ���ж��Ժ�ǿ���ԣ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��