��Ŀ����
 ��������ͼ��ʾ��װ����ȡ����������
��������ͼ��ʾ��װ����ȡ������������1��д����ȡ���������Ļ�ѧ����ʽ��
��2��װ����ͨ�����ĵ���Ҫ�嵽����̼������ҺҺ���ϣ������ܲ���Һ�����£�Ŀ���Ƿ�ֹ
��3��ʵ����ȡ����������������
���㣺������������ȡ
ר�⣺ʵ����
��������1��������Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��2�����ܲ��ܲ���Һ���µ�ԭ���Ƿ�ֹ������ˮ���紦�۲쵽�����ݲ��������ݵ���Ҫ�ɷ��������̼�����Ʒ�Ӧ���ɵĶ�����̼��
��3�����뻥�����ܵ�Һ����÷�Һ�ķ������룬�������������뱥��̼����Һ�廥�����ܣ��ҳ�������������ΪҺ�壬�ݴ˽�ɣ�
��2�����ܲ��ܲ���Һ���µ�ԭ���Ƿ�ֹ������ˮ���紦�۲쵽�����ݲ��������ݵ���Ҫ�ɷ��������̼�����Ʒ�Ӧ���ɵĶ�����̼��
��3�����뻥�����ܵ�Һ����÷�Һ�ķ������룬�������������뱥��̼����Һ�廥�����ܣ��ҳ�������������ΪҺ�壬�ݴ˽�ɣ�
���
�⣺��1��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴Ӧ���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
��2���ӷ��������ᡢ�Ҵ�������ˮ���������¶˹ܿڲ��ܽ���Һ�������Һ�з�ֹ����������Ҫ���ڱ���̼������Һ��Һ���ϣ�����Һ���»ᷢ�������������ӷ����Ʊ������������������ᣬ������̼���Ʒ�Ӧ���ɶ�����̼����ѧ��Ӧ����ʽΪ��2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O���ʴ�Ϊ����ֹ������2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��
��3�����뻥�����ܵ�Һ����÷�Һ�ķ��������Һ����Ϊ��������������Һ̬���ʣ����뱥�͵�̼������Һ�������ܣ��ʿ��Բ�ȡ�˷������ʴ�Ϊ����Һ����������������Һ̬���ʣ����뱥�͵�̼������Һ�������ܣ�
| Ũ���� |
| �� |
��2���ӷ��������ᡢ�Ҵ�������ˮ���������¶˹ܿڲ��ܽ���Һ�������Һ�з�ֹ����������Ҫ���ڱ���̼������Һ��Һ���ϣ�����Һ���»ᷢ�������������ӷ����Ʊ������������������ᣬ������̼���Ʒ�Ӧ���ɶ�����̼����ѧ��Ӧ����ʽΪ��2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O���ʴ�Ϊ����ֹ������2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��
��3�����뻥�����ܵ�Һ����÷�Һ�ķ��������Һ����Ϊ��������������Һ̬���ʣ����뱥�͵�̼������Һ�������ܣ��ʿ��Բ�ȡ�˷������ʴ�Ϊ����Һ����������������Һ̬���ʣ����뱥�͵�̼������Һ�������ܣ�
���������⿼�������������Ʊ�����Ŀ�Ѷ��еȣ�ע�����ⱥ��̼������Һ�������Լ�������Ӧ�Ļ���������������ѧ���������������������Ӧ����ѧ֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A��1s22s12p1��ʾ���Ǽ���̬ԭ�ӵĵ����Ų� |
| B������ͬһ����ϵĵ��ӵ��˶�״̬��ȫ��ͬ |
| C���ں�4 mol Si-O���Ķ������辧���У���ԭ�ӵ���ĿΪ4NA |
| D������衢�����ʾ�������Ӧԭ��ֱ�ӹ��ɵ�ԭ�Ӿ��� |
���������������������Ӧ���Ȼ�ѧ����ʽ��25�棬101kPa����
��C4H10��g��+
O2��g���T4CO2��g��+5H2O��l����H=-2 878kJ?mol-1
��C4H10��g��+
O2��g���T4CO2��g��+5H2O��g����H=-2 658kJ?mol-1
��C4H10��g��+
O2��g���T4CO��g��+5H2O��l����H=-1 746kJ?mol-1
��C4H10��g��+
O2��g���T4CO��g��+5H2O��g����H=-1 526kJ?mol-1
�ɴ��жϣ��������ȼ�����ǣ�������
��C4H10��g��+
| 13 |
| 2 |
��C4H10��g��+
| 13 |
| 2 |
��C4H10��g��+
| 9 |
| 2 |
��C4H10��g��+
| 9 |
| 2 |
�ɴ��жϣ��������ȼ�����ǣ�������
| A����H=-2 878 kJ?mol-1 |
| B����H=-2 658 kJ?mol- |
| C����H=-1 746 kJ?mol-1 |
| D����H=-1 526 kJ?mol-1 |
��ѧ����ʽ�ɼ���������Ԫ�ؼ��仯��������ʣ���֪��
������ԭ��Ӧ��2FeCl3+2HI�T2FeCl2+I2+2HCl��
2Co��OH��3+6HCl�T2CoCl2+Cl2��+6H2O
2Fe��OH��2+I2+2KOH�T2Fe��OH��3+2KI��
3I2+6KOH�T5KI+KIO3+3H2O
���ֽⷴӦ��2HSCN+K2CO3�T2KSCN+CO2��+H2O�� KCN+CO2+H2O�THCN+KHCO3
�ȷֽⷴӦ��4NaClO
3NaCl+NaClO4��NaClO4
NaCl+2O2��
����˵������ȷ�ǣ�������
������ԭ��Ӧ��2FeCl3+2HI�T2FeCl2+I2+2HCl��
2Co��OH��3+6HCl�T2CoCl2+Cl2��+6H2O
2Fe��OH��2+I2+2KOH�T2Fe��OH��3+2KI��
3I2+6KOH�T5KI+KIO3+3H2O
���ֽⷴӦ��2HSCN+K2CO3�T2KSCN+CO2��+H2O�� KCN+CO2+H2O�THCN+KHCO3
�ȷֽⷴӦ��4NaClO
| ||
| ||
����˵������ȷ�ǣ�������
| A�����ԣ�ˮ��Һ����HSCN��H2CO3��HCN |
| B����ԭ�ԣ�������Һ����Fe��OH��2��I2��KIO3 |
| C�����ȶ��ԣ�NaCl��NaClO4��NaClO |
| D�������ԣ�������Һ����FeCl3��Co��OH��3��I2 |
 ��ͼ��ij��ѧѧϰС������̽������ˮ��Ӧ��ʵ��װ�ã���װ�ÿ��Լ���Ӧ���ռ�������������һ�壮ͼ���ƿ����ô�ͷ���ס�ģ�����գ�
��ͼ��ij��ѧѧϰС������̽������ˮ��Ӧ��ʵ��װ�ã���װ�ÿ��Լ���Ӧ���ռ�������������һ�壮ͼ���ƿ����ô�ͷ���ס�ģ�����գ�
 ��Դ����������ͷ�չ����Ҫ֧����̼Ԫ�صĵ��ʼ������������������������Ҫ��Դ���ʣ�
��Դ����������ͷ�չ����Ҫ֧����̼Ԫ�صĵ��ʼ������������������������Ҫ��Դ���ʣ�
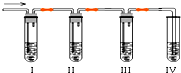 ��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺