��Ŀ����
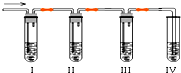 ��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺��1����װ�ÿ�ʢ�ŵ��Լ��ǣ���
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
��2����˵��SO2������ڵ�������
��3��ʹ��װ��II��Ŀ����
��4��ʹ��װ��III��Ŀ����
��5��ȷ��������ϩ��������
��6�������Լ�����
���㣺����ʵ�鷽�������
ר�⣺ʵ�������
��������������ļ�����Ʒ����Һ����ϩ�ļ����ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ��ȥSO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ��Ȼ��ش����⣮
��1�����������Ƿ���ڿ���Ʒ����Һ���飻������ϩ��������ˮ��������������Һ����ϩ�Ͷ���������ʹ��ˮ��������������Һ��ɫ����ϩ����ˮ�����ӳɷ�Ӧʹ��ˮ��ɫ��CH2=CH2+Br2��CH2Br-CH2Br����ϩ�����Ը����������ʹ����ɫ��������������ˮ�����Ը�����ط���������ԭ��Ӧ��5SO2+2KMnO4+2H2O�TK2SO4+2MnSO4+2H2SO4��SO2+Br2+H2O�TH2SO4+2HBr����ϩ����NaOH��Һ��Ӧ����������������Ӧ��SO2+2NaOH=Na2SO3+H2O��������ϩ�ļ���Ӧ�����ų�SO2�ĸ��ź���У�ѡͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ���ͨ��NaOH��Һ��ȥSO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫʵ�������ϩ��
��2��ͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ�
��3��SO2+Br2+H2O�TH2SO4+2HBr����ȥ�����������壬���������ϩ��ʵ�飻
��4����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���
��5���ø������������Һ��ɫʵ�������ϩ��
��6������������ˮ������ϩ�Ĵ��ڣ�
��1�����������Ƿ���ڿ���Ʒ����Һ���飻������ϩ��������ˮ��������������Һ����ϩ�Ͷ���������ʹ��ˮ��������������Һ��ɫ����ϩ����ˮ�����ӳɷ�Ӧʹ��ˮ��ɫ��CH2=CH2+Br2��CH2Br-CH2Br����ϩ�����Ը����������ʹ����ɫ��������������ˮ�����Ը�����ط���������ԭ��Ӧ��5SO2+2KMnO4+2H2O�TK2SO4+2MnSO4+2H2SO4��SO2+Br2+H2O�TH2SO4+2HBr����ϩ����NaOH��Һ��Ӧ����������������Ӧ��SO2+2NaOH=Na2SO3+H2O��������ϩ�ļ���Ӧ�����ų�SO2�ĸ��ź���У�ѡͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ���ͨ��NaOH��Һ��ȥSO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫʵ�������ϩ��
��2��ͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ�
��3��SO2+Br2+H2O�TH2SO4+2HBr����ȥ�����������壬���������ϩ��ʵ�飻
��4����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���
��5���ø������������Һ��ɫʵ�������ϩ��
��6������������ˮ������ϩ�Ĵ��ڣ�
���
�⣺��1����ϩ����NaOH��Һ��Ӧ����������������Ӧ��SO2+2NaOH=Na2SO3+H2O�������������Ƿ���ڿ���Ʒ����Һ���飮��ϩ�ļ���Ӧ�����ų�SO2�ĸ��ź���У�ѡͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ���ͨ��NaOH��Һ��ȥSO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫʵ�������ϩ��
��װ�â���������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ�â��Թ�װ��NaOH��Һ��ȥSO2��װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ�â�ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��A��B��A��D��
��2�����������Ƿ���ڿ���Ʒ����Һ���飬Ʒ����Һ��ɫ˵�����ж�������
�ʴ�Ϊ��װ�â���Ʒ����Һ��ɫ��
��3����ϩ�Ͷ���������ʹ��ˮ��������������Һ��ɫ����������Ĵ���Ӱ����ϩ�ļ��飬�ʼ�����ϩʱӦ�ȳ�ȥ��������
�ʴ�Ϊ����ȥ�����������壬���������ϩ��ʵ�飻
��4��ͨ��NaOH��Һ��ȥSO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���
�ʴ�Ϊ��������������Ƿ������
��5������ø������������Һ��ɫʵ�������ϩ��װ�â��е����Ը��������Һ��ɫ��˵��������ϩ��
�ʴ�Ϊ��װ�â��е�Ʒ����Һ����ɫ��װ�â��е����Ը��������Һ��ɫ��
��6������������ˮ������ϩ�Ĵ��ڣ����ڵ���������ˮ��ɫ��
�ʴ�Ϊ����ˮ����ˮ��ɫ��
��װ�â���������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ�â��Թ�װ��NaOH��Һ��ȥSO2��װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ�â�ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��A��B��A��D��
��2�����������Ƿ���ڿ���Ʒ����Һ���飬Ʒ����Һ��ɫ˵�����ж�������
�ʴ�Ϊ��װ�â���Ʒ����Һ��ɫ��
��3����ϩ�Ͷ���������ʹ��ˮ��������������Һ��ɫ����������Ĵ���Ӱ����ϩ�ļ��飬�ʼ�����ϩʱӦ�ȳ�ȥ��������
�ʴ�Ϊ����ȥ�����������壬���������ϩ��ʵ�飻
��4��ͨ��NaOH��Һ��ȥSO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���
�ʴ�Ϊ��������������Ƿ������
��5������ø������������Һ��ɫʵ�������ϩ��װ�â��е����Ը��������Һ��ɫ��˵��������ϩ��
�ʴ�Ϊ��װ�â��е�Ʒ����Һ����ɫ��װ�â��е����Ը��������Һ��ɫ��
��6������������ˮ������ϩ�Ĵ��ڣ����ڵ���������ˮ��ɫ��
�ʴ�Ϊ����ˮ����ˮ��ɫ��
���������⿼����ϩ�Ļ�ѧ���ʡ��Ʊ��Լ���������ļ��飬ע��ʵ����Ⱥ�˳����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ
����������ȼ��ʱ�ĵõ�һ�ֱ��������ᄃ�壬��ṹ��ͼ��ʾ���й�˵����ȷ���ǣ�������

| A���þ����������Ӿ��� |
| B������Ļ�ѧʽΪBa2O2 |
| C���þ��徧���ṹ��CsCl���� |
| D����ÿ��Ba2+��������������Ba2+����12�� |
ij����ԭ�ӵĺ������ĸ��ܲ㣬�����ܲ���1�����ӣ���ԭ�Ӻ��ڵ�����������Ϊ��������
| A��24 | B��18 | C��19 | D��29 |
a g Mg��Al�Ͻ���ȫ�ܽ���C1 mol?L-1��V1L HCl��Һ�У�����b g H2������Ӧ�����Һ�м���C2mol?L-1��V2L NaOH��Һ��ǡ��ʹ�����ﵽ���ֵ���ҳ�������Ϊd g�����й�ϵ������ǣ�������
A����Ϊ
| ||
B��C1=
| ||
| C��d=a+17b | ||
| D���������Ӧ��ʣ������Ϊ��C1V1-b��mol |
�л���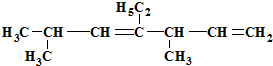 �������ǣ�������
�������ǣ�������
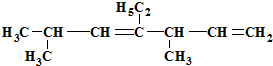 �������ǣ�������
�������ǣ�������| A��2��5-����-4-�һ�-3��6-����ϩ |
| B��1��1��4-����-3-�һ�-2��5-����ϩ |
| C��3��6-����-4-�һ�-1��4-����ϩ |
| D��2��5-����-4-�һ�-3��6-����ϩ |
���������������ȷ���ǣ�������
| A��Ũ�������ǿ�����ԣ�ϡ������������ |
| B��Ũ����ε������ϣ�������ɰ�ɫ��ĩ��������Ũ�������ˮ�� |
| C��ϡ��Ũ����ʱӦ��Ũ���������ձ���������ע��ʢ��ˮ���ձ��У������Ͻ��� |
| D��Ũ������ͭ�ķ�Ӧ�У�Ũ���������ǿ������ |
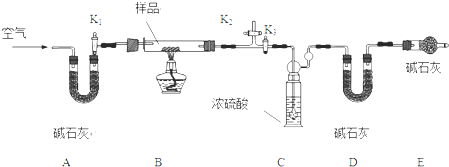
 ��������ͼ��ʾ��װ����ȡ����������
��������ͼ��ʾ��װ����ȡ���������� ���ϳ�·�����£�
���ϳ�·�����£�

 ��R-����������
��R-����������