��Ŀ����
6��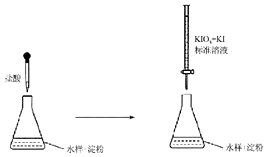 ������������������Ӧ�ù㷺����ѧʹ�ö����彡�����������������ش�
������������������Ӧ�ù㷺����ѧʹ�ö����彡�����������������ش���1�����������һ������SO2 ���Է�ֹ��Һ��������Ӧ����SO2 �Ļ�ԭ�ԣ�
��2��ijˮ������Ԫ����Ҫ��S2O32-��ʽ���ڣ������������£������ӻᵼ��ˮ�����������Ũ������ԭ���������������£���Һ�е�H+��S2O32-������Ӧ��H++S2O32-=SO2+S��+H2O��SO2����ˮ��������ӦSO2+H2O?H2SO3��ʹˮ����������Ũ������
��3��ʵ���Ҳ��õζ����ⶨijˮ�����������κ�����
�ٵζ�ʱ��KIO3 ��KI ��������I2����ɲ���ƽ�������ӷ���ʽ��1IO3-+5I-+6H+=3I2+3H2O
�ڷ�Ӧ������I2������������SO32-��S2O32-�������������������ɫ���������ڹ۲�ζ��յ㣮
�۵ζ��յ�ʱ��100mLˮ��������xmL����Һ��������1mL����Һ�൱��SO32-������1g�����ˮ����SO32-�ĺ���Ϊ104xmg/L��
���� ��1�����������һ������SO2 ���Է�ֹ��Һ������������������
��2�������������£������ӻᵼ��ˮ�����������Ũ��������������ԭ��Ӧ�йأ�
��3����IԪ�صĻ��ϼ���+5�۽���Ϊ0��IԪ�صĻ��ϼ���-1������Ϊ0��
�ڵⵥ�ʾ��������ԣ��ҵ������������
������1mL����Һ�൱��SO32-������1g��100mLˮ���൱��SO32-������xg���Դ˼��㣮
��� �⣺��1�����������һ������SO2 ���Է�ֹ��Һ�����������������������仹ԭ�ԣ��ʴ�Ϊ����ԭ��
��2��ijˮ������Ԫ����Ҫ��S2O32-��ʽ���ڣ������������£������ӻᵼ��ˮ�����������Ũ������ԭ���������������£���Һ�е�H+��S2O32-������Ӧ��H++S2O32-=SO2+S��+H2O��SO2����ˮ��������ӦSO2+H2O?H2SO3��ʹˮ����������Ũ������
�ʴ�Ϊ�������������£���Һ�е�H+��S2O32-������Ӧ��H++S2O32-=SO2+S��+H2O��SO2����ˮ��������ӦSO2+H2O?H2SO3��ʹˮ����������Ũ������
��3����IԪ�صĻ��ϼ���+5�۽���Ϊ0��IԪ�صĻ��ϼ���-1������Ϊ0���ɵ��ӡ�����غ��֪���ӷ�ӦΪIO3-+5I-+6H+=3I2+3H2O��
�ʴ�Ϊ��1��5��6H+��3��3��
�ڷ�Ӧ������I2������������SO32-��S2O32-�������������������ɫ���������ڹ۲�ζ��յ㣬
�ʴ�Ϊ������SO32-��S2O32-�������������������ɫ���������ڹ۲�ζ��յ㣻
������1mL����Һ�൱��SO32-������1g��100mLˮ���൱��SO32-������xg�����ˮ����SO32-�ĺ���Ϊ$\frac{xg��1000mg/g}{0.1L}$=104xmg/L���ʴ�Ϊ��104x��
���� ���⿼�����ʵ����ʼ������ⶨ��Ϊ��Ƶ���㣬����������ԭ��Ӧ���ⶨԭ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע�����Ϊ�����ѵ㣬��Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1 mol CO2��1 mol CO��ռ�������ͬ��������������ͬ | |
| B�� | 1 g CO��1 g CO2��ռ�������ͬ��������������ͬ | |
| C�� | ���κ�����£�1 mol CO2��64 g SO2�����з�������ԭ����������ͬ | |
| D�� | ij�������������ӵ����������������ʱ�״�������ԼΪ22.4 L |
| A�� | ƽ����ϵ��C���������Ϊ$\frac{1}{9}$ | B�� | ƽ��ʱA��Ũ��Ϊ2.4mol/L | ||
| C�� | D��ƽ������Ϊ0.32mol/L•min | D�� | B��ת����Ϊ20% |
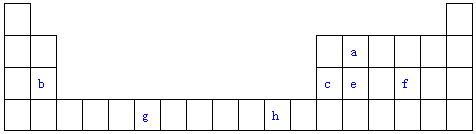
| Ԫ�� | �����Ϣ |
| A | ��̬ԭ�ӵļ۵����Ų�ʽΪnSnnPn |
| B | ��̬ԭ���е�δ�ɶԵ�������ͬ���������� |
| C | �����������ǵ��Ӳ�����3�� |
| D | �������ǵ�������Ԫ�������Ӱ뾶��С�� |
| E | �۵��Ӳ��е�δ�ɶԵ�����Ϊ4 |
��1��д��DԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ3s23p1��E2+�۵��ӵĹ����ʾʽ
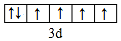 ��
����2����5��Ԫ���е縺������Ԫ����O����Ԫ�ط��ţ���A��B��C����Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��C����Ԫ�ط��ţ�
��3��B��C��D�ļ����ӵİ뾶�ɴ�С��˳��ΪN3-��O2-��Al3+�������ӷ��ű�ʾ��
��4��д��C�ĺ�����18�����ӵ��⻯��ĵ���ʽ
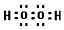 ��
��  ��ͼ��ʾ�������ձ��е缫�õ����������ĸ��缫�ֱ�ΪMg��Al��Pt��C�����պϿ���S�����±�����ȷ���ǣ�������
��ͼ��ʾ�������ձ��е缫�õ����������ĸ��缫�ֱ�ΪMg��Al��Pt��C�����պϿ���S�����±�����ȷ���ǣ�������| A�� | ������ָ�벻����ƫת | |
| B�� | Al��Pt������H2���� | |
| C�� | �׳�pH�����ҳ�pH��С | |
| D�� | Mg��C�������ɵ�������һ�������¿���ǡ����ȫ��Ӧ |