��Ŀ����
2���±��г��ˢ١������Ԫ�������ڱ��е�λ�ã�| ��A | ��A | |||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | ||||
��1��Ԫ�آܵ�����������Ԫ�آ������ڱ�������λ�õڶ����ڵڢ�A��
��2������̬�⻯����ȶ���������ǿ��˳�����У��ޢܢߵ��⻯���ȶ���PH3��H2S��H2O ��д�⻯��Ļ�ѧʽ����
��3��Ԫ�آߵ�ԭ�ӽṹʾ��ͼ��
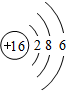 ��
����4��д��ʵ������ȡ�۵��⻯��Ļ�ѧ��Ӧ����ʽ2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O
��5���õ���ʽ��ʾ����ᷴӦ�õ��Ļ�������γɹ���
 ��
��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪMg����ΪNa����ΪP����ΪS����ΪNe����ΪCl��
��1����λ�ڵڶ����ڵڢ�A�壻
��2���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ���
��3��S��ԭ������Ϊ16��
��4������������Ʊ�������
��5������ᷴӦ�õ��Ļ�����ΪHCl��Ϊ���ۻ����
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪMg����ΪNa����ΪP����ΪS����ΪNe����ΪCl��
��1��Ԫ�آܵ�����������λ�ڵڶ����ڵڢ�A�壬�ʴ�Ϊ�������ڶ����ڵڢ�A�壻
��2���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ����ޢܢߵ��⻯���ȶ���ΪPH3��H2S��H2O���ʴ�Ϊ��PH3��H2S��H2O��
��3��S��ԭ������Ϊ16��ԭ�ӽṹʾ��ͼΪ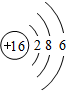 ���ʴ�Ϊ��
���ʴ�Ϊ��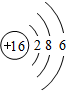 ��
��
��4������������Ʊ���������ӦΪ2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��5������ᷴӦ�õ��Ļ�����ΪHCl��Ϊ���ۻ�����õ���ʽ��ʾ�γɹ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼��λ�á��ṹ�����ʵ�Ӧ�ã�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʡ�Ԫ�ػ�����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�ػ����P��ѧ�����Ӧ�ã���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� |  ��C2H5OH ��C2H5OH | B�� |  ��C2H518OH ��C2H518OH | ||
| C�� |  ��CH3CH2CH218OH ��CH3CH2CH218OH | D�� |  ��C2H5OH ��C2H5OH |
| A�� | ��ȩ�����ᡢ����������Ҵ�����ʹ���Ը��������Һ��ɫ | |
| B�� | ��ͬ�����������ʵ������Ҵ����Ҷ������������зֱ���������Ľ����Ʋ������������֮����2��3��6 | |
| C�� | ���Ӻ���ȩ������ʹ��ˮ��ɫ���������ķ�Ӧ���Ͳ�ͬ | |
| D�� | ��ˮ�ȿɼ�����������ϩ���ֿɳ�ȥ�����е���ϩ���õ����������� |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� |
��1������Ԫ���У�����������Ӧˮ����������ǿ����HClO4��������ǿ����KOH�������Ե���Al��OH��3���ѧʽ��
��2���ۡ��ܡ�������Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������ΪK��Na��Mg����Ԫ�ط��ţ����ۡ��ܡ��ݡ��ޡ��ߡ�������Ԫ�ض�Ӧ���Ӱ뾶��С��ΪAl3+�������ӷ��ţ�
��3����Ҫ��д�������������ʵĵ���ʽ��������������Ķ�Ӧ��ˮ����
 ����H�͢��γɵ�ԭ����֮��Ϊ1��1������
����H�͢��γɵ�ԭ����֮��Ϊ1��1������ ��
����4���ٵ��⻯���ڳ�������ᷢ����Ӧ�Ļ�ѧ����ʽΪ2K+2H2O�T2KOH+H2����������Һ��ݵ�����������Ӧˮ���ﷴӦ�����ӷ���ʽΪAl��OH��3+OH-�TAlO2-+2H2O��
| ѡ�� | ʵ����� | ��������ۣ���װ����;�� |
| A | ij��Һ����ŨNaOH��Һ���ȣ����Թܿڷ�һƬʪ��ĺ�ɫʯ����ֽ | ��ֽ������˵��NH3�Ǽ� |
| B |  | �������ڱȽ�Fe3+��I2��Cl2��������ǿ�� |
| C | 2mL 2% CuSO4�м�4��6��2% NaOH��Һ�������0.5mL X��Һ��������� | δ����ש��ɫ������˵��X������ȩ�� |
| D |  | ��������HCl�����ܷ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ${\;}_{53}^{131}$I��̬ԭ�ӵĵ����˶�״̬��78�� | |
| B�� | �������${\;}_{53}^{131}$I | |
| C�� | ${\;}_{53}^{131}$I�����������������IJ���25 | |
| D�� | ${\;}_{53}^{131}$I�з����Զ�${\;}_{53}^{126}$Iû�У������Ǻ�������Ų���ͬ |
| A�� | C�ķ�Ӧ������B������������� | |
| B�� | ��λʱ��������2n mol A��ͬʱ����3n mol B | |
| C�� | A��B��C��Ũ�Ȳ��ٱ仯 | |
| D�� | A��B��C��Ũ��֮��Ϊ1��3��2 |
 C��Be��Cl��Fe��Ԫ�ؼ��仯��������Ҫ��Ӧ�ã�
C��Be��Cl��Fe��Ԫ�ؼ��仯��������Ҫ��Ӧ�ã�