��Ŀ����
20��ijͭ��ʯ������ͭ��������ͭ����������������ʯ��SiO2�����ֲ���������ӿ�ʯ����ȡͭ���乤������ͼ���£�����ͭ����ȡ��ͭ��ˮ������л���Ĺ��̣��ͷ���ȡ��ͭ���л������ˮ��Ĺ��̣����ִ�ʪ����ͭ����Ҫ�����ֶΣ�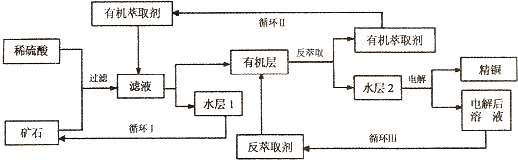
��֪����Cu2O+2H+�TCu2++Cu+H2O���ڵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ�� �۷���ȡ���ˮ��2������ͭ��Һ��
�ش��������⣺
��1����ʯ��ϡ���ᴦ�������з�����Ӧ�����ӷ���ʽΪ��Cu2O+2H+�TCu2++Cu+H2OFe2O3+6H+�T2Fe3++3H2O��Cu+2Fe3+�T2Fe2++Cu2+��д����2����
��2�����л����ˮ�����IJ��������з�Һ��ʵ������ɴ˲������õ���һ����Ҫ�����Ƿ�Һ©����
��3����ѭ��I�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�������ξ��壬�þ���Ļ�ѧʽ��FeSO4•7H2O��
��4��д���������У����缫���Ƕ��Ե缫������������Ӧ�ĵ缫��ӦʽCu2++2e-=Cu
��5����ѭ�����з���ȡ������Ҫ�ɷ���H2SO4��
���� ��1������ͭ�����������������Ժ�ǿ�ᷢ����Ӧ�����κ�ˮ������ͭ��������֮�䷢��������ԭ��Ӧ��
��2���������ֻ������ܵ�Һ���÷�Һ��������Һ������Ҫ�õ��������Ƿ�Һ©����
��3��һ�������£���������������FeSO4•7H2O����ʽ���ڣ�
��4���������ͭʱ����������ͭ���ӷ����õ��ӻ�ԭ����Ӧ��
��5������ѭ��ͼ�ҳ�ѭ����ķ���ȡ����
��� �⣺��1������ͭ�����������������Ժ�ǿ�ᷢ����Ӧ�����κ�ˮ��CuO+2H+�TCu2++H2O��Fe2O3+6H+�T2Fe3++3H2O������ͭ��������֮�䷢��������ԭ��Ӧ����Cu+2Fe3+�T2Fe2++Cu2+��
�ʴ�Ϊ��Fe2O3+6H+�T2Fe3++3H2O��Cu+2Fe3+�T2Fe2++Cu2+��
��2���������ֻ������ܵ�Һ���÷�Һ���������Խ��л����ˮ�����IJ��������з�Һ����Һ������Ҫ�õ��������Ƿ�Һ©����
�ʴ�Ϊ����Һ����Һ©����
��3����ѭ�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�ľ���FeSO4•7H2O��
�ʴ�Ϊ��FeSO4•7H2O��
��4���������ͭʱ����������ͭ���ӷ����õ��ӻ�ԭ����Ӧ����Cu2++2e-=Cu��
�ʴ�Ϊ��Cu2++2e-=Cu��
��5����ѭ�����з���ȡ������Ҫ�ɷ������ᣬ�ʴ�Ϊ��H2SO4��
���� ���⿼����ͭ���仯��������ʣ��������ǿ���Ƚϡ����ʵķ�����ᴿ��������ԭ��Ӧ�ļ��㣬�漰������ӷ�����ʽ����д���Ѷ��еȣ���Ҫ�����������ȡ���е���Ϣ��


| A�� | ���±��ͨ��Cl2��Ϊ����ȡ�� | |
| B�� | ���οɲ��ó��Ӻ��ؽᾧ�ȹ����ᴿ | |
| C�� | ������һ�����ÿ�����ˮ�������������壬����SO2���仹ԭ���� | |
| D�� | ��ҵ�����г�ѡ��NaOH��Ϊ������ |
| A�� | �����������ְ�ɫ����ֱ����1 mol/L�������У����������ݲ��� | |
| B�� | �ֱ�ȡ�����Թ��м��ȣ������ܲ���������ͨ�����ʯ��ˮ���۲����ް�ɫ���� | |
| C�� | �ֱ�ȡ�������Һ���μ�BaCl2��Һ���۲����ް�ɫ���� | |
| D�� | �ֱ������Һ���ò�˿պȡ��Һ�ھƾ��ƻ��������գ��۲�������ɫ |
| A�� | ����Ԫ�� | B�� | ��A��Ԫ�� | C�� | ±���� | D�� | ��A��Ԫ�� |
| pH | 6.5��6.8 |
| Ca2+��Mg2+��Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/L |
Դˮ��������$\stackrel{��ʯ��}{��}$һ��������$��_{ͨ��CO_{2}}^{���ۼ���}$����������$\stackrel{����A}{��}$���˳ء�����ˮ
��1��Դˮ�к�Ca2+��Mg2+��HCO3-��Cl-�ȣ�����ʯ�Һ�����Ca��OH��2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽMg2++OH-=Mg��OH��2����
��2�����ۼ���ȥ������������Ĺ��̢ۣ���д��ţ���ѡ���۷֣�
��ֻ���������� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ����
FeSO4.7H2O�dz��õ����ۼ�������ˮ����������Fe��OH��3������
��3��ͨ�������̼��Ŀ���ǽ�Ca2+��ȥ�͵�����Һ�е�pH�ﵽ����ˮ��������
��4������A��������ɱ�����������������ǻ�������A��ˮ��Ӧ�IJ������ǿ�����ԣ�
��5�����������У��ٺ͢ۿ�����Ϊ����A�Ĵ���Ʒ������д��ţ���ѡ���۷֣�
��Ca��ClO��2 ��NH3��Һ�� ��K2FeO4 ��SO2��
| X | Y | |
| Z | W |
 ��
����2��A��B��������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪAl��OH��3+NaOH�TNaAlO2+2H2O��
��3��AW3�����ھ�ˮ����ԭ����Al3++3H2O?Al��OH��3�����壩+3H+�����������ӷ���ʽ��ʾ��
��4����ҵ�ϳ�X�ļ���̬�⻯���Ƿ��ȷ�Ӧ�����д�ʩ�м�����߷�Ӧ���ʣ��������ԭ��ת���ʵ���d��
a�������¶ȡ���������������������������b���������
c���� X�ļ���̬�⻯�Pʱ���롡������d������Ӧ��ϵ��ѹǿ
��5����״���£�2.24 L X�ļ���̬�⻯�ﱻ200 mL 1 mol•L-1 X������������Ӧ��ˮ������Һ���պ�������Һ������Ũ�ȴӴ�С��˳���ǣ������ӷ��ű�ʾ��c��NO3-����c��H+����c��NH4+����c��OH-����
��6��WY2��ɱ��������ͬʱ���ɽ��綾�軯�����������������ȥ��д���� WY2���е�9.9�棩������ȥCN-�����ӷ���ʽ2ClO2+2CN-�TN2+2CO2+2Cl-��
��Ϣ�٣�ԭ�Ӱ뾶��С��A��B��C��D
��Ϣ�ڣ�����Ԫ��֮���γɵ�ij���ַ��ӵı���ģ�ͼ��������ʣ�
 |  |  |
| �ǵ��������������֮һ������ΪҺ̬���ǰ��������������������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��� | ��ɫ����ζ����ȼ����21���͵���Ҫ��Դ | ���ᣬ��ǿ�����ԣ�������������ɱ�� |
��1���ס��ҡ����к��еĹ�ͬԪ�����⣨�����ƣ���
��2��BԪ�������ڱ��е�λ��Ϊ��2���ڢ�A�壮
��3������Ԫ�ص�ԭ��M����һ��δ�ɶ�p���ӵ���Cl����Ԫ�ط��ţ���
��4�����ĵ���ʽΪ
 ������SO2ˮ��Һ�ɷ���������ԭ��Ӧ����������ǿ�ᣬ��ѧ��Ӧ����ʽΪHClO+H2O+SO2=H2SO4+HCl��
������SO2ˮ��Һ�ɷ���������ԭ��Ӧ����������ǿ�ᣬ��ѧ��Ӧ����ʽΪHClO+H2O+SO2=H2SO4+HCl�� | A�� | ֻ������ | B�� | ֻ���Ǽ� | ||
| C�� | ��������Ҳ�����Ǽ� | D�� | ֻ������ |