��Ŀ����
��֪25�桢101kPa�£�ϡ��ǿ����ǿ����Һ��Ӧ���к���Ϊ57.3kJ/mol��
��1��д����ʾ�������ռ���Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ�� ��
��2��ijʵ��С���� 0.25mol/L��ϡ������Һ�� 0.55mol/L��ϡ�ռ���Һ�����к��ȵIJⶨ��
I������0.25mol/LH2SO4��Һ
����ʵ���д�ԼҪʹ��245ml 0.25mol/L H2SO4��Һ����Ҫ����Ͳ��ȡ�ܶ�Ϊ1.8g/cm3��������������Ϊ98%��Ũ���� mL
�ڴ���ͼ��ѡ������0.25mol/L H2SO4��Һ����Ҫ������������ĸ��
II��ʵ��С����ϡ������ϡ�ռ���Һ�ⶨ�к���װ����ͼ��

�ٸ�װ��������k�������� ��װ������һ�������� �������
�ô���װ�òⶨ������к�����ֵ�� ���ƫ�ߡ���ƫ�͡�����Ӱ�족��
�ڸ�С��ͬѧ��ʱ�������Ⲣ�������ѡ��50mL 0.25mol/L��ϡ
������50mL 0.55mol/L��ϡ�ռ���Һ�������飬ʵ���в�á�t=3.2�棬
�跴Ӧǰ�������ռ���Һ���ܶȾ�Ϊ1g/cm3���кͺ���Һ�ı�����Ϊ4.18J/��g?�棩����ô��С��ⶨ�к��ȣ���H= ��
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��
a����ȡH2SO4��Һ��NaOH��Һǰ�ô���Һ����Ͳ��������ϴ��
b������H2SO4��Һ�¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�ֱ�Ӳ�NaOH��Һ���¶ȣ�
c���ֶ�ΰ�NaOH��Һ����ʢ��H2SO4��Һ��С�ձ��У�
d����ȡH2SO4��Һʱ���Ӷ�����
e���ҿ�Ӳֽ���ò��������裮
��1��д����ʾ�������ռ���Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ��
��2��ijʵ��С���� 0.25mol/L��ϡ������Һ�� 0.55mol/L��ϡ�ռ���Һ�����к��ȵIJⶨ��
I������0.25mol/LH2SO4��Һ
����ʵ���д�ԼҪʹ��245ml 0.25mol/L H2SO4��Һ����Ҫ����Ͳ��ȡ�ܶ�Ϊ1.8g/cm3��������������Ϊ98%��Ũ����
�ڴ���ͼ��ѡ������0.25mol/L H2SO4��Һ����Ҫ������������ĸ��
| ���� | ������ƽ�������룩 | С�ձ� | ����ƿ | ������ | ҩ�� | ��Ͳ | ��ͷ�ι� |
| ���� |  |
 |
 |
 |
 |
 |
 |
| ��� | a | b | c | d | e | f | g |

�ٸ�װ��������k��������
�ô���װ�òⶨ������к�����ֵ��
�ڸ�С��ͬѧ��ʱ�������Ⲣ�������ѡ��50mL 0.25mol/L��ϡ
������50mL 0.55mol/L��ϡ�ռ���Һ�������飬ʵ���в�á�t=3.2�棬
�跴Ӧǰ�������ռ���Һ���ܶȾ�Ϊ1g/cm3���кͺ���Һ�ı�����Ϊ4.18J/��g?�棩����ô��С��ⶨ�к��ȣ���H=
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��
a����ȡH2SO4��Һ��NaOH��Һǰ�ô���Һ����Ͳ��������ϴ��
b������H2SO4��Һ�¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�ֱ�Ӳ�NaOH��Һ���¶ȣ�
c���ֶ�ΰ�NaOH��Һ����ʢ��H2SO4��Һ��С�ձ��У�
d����ȡH2SO4��Һʱ���Ӷ�����
e���ҿ�Ӳֽ���ò��������裮
���㣺�Ȼ�ѧ����ʽ,�к��ȵIJⶨ
ר�⣺��ѧ��Ӧ�е������仯
��������1�������к�����ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų�����������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ�д���Ȼ�ѧ����ʽ����2�����ŨH2SO4�����ʵ���Ũ��C=���ٸ�����Һϡ��ǰ�����ʵ��������������Ũ���������� ��ʵ����������Һ��������ֱ��Ǽ��㡢��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ����ݸ���������Ҫʹ�õ�������
��ٸ���������װ��ͼ����֪k�����ƺ�ȱ��Ӳֽ�壮���ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ��
�ڸ���Q=m?c?��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��a����Ͳ��ȡ��Һǰ��Ҫ��ϴ���������ϡ�ͣ�
b�ᵼ��ʵ�����к��ȵ���ֵƫС��c�������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�d��ȡH2SO4��Һ�����ʱ���Ӷ������ᵼ��������H2SO4���ƫ�ų�������ƫ�ߣ�e�����������ɢʧ������ͨ�����β������������裬
��ٸ���������װ��ͼ����֪k�����ƺ�ȱ��Ӳֽ�壮���ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ��
�ڸ���Q=m?c?��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��a����Ͳ��ȡ��Һǰ��Ҫ��ϴ���������ϡ�ͣ�
b�ᵼ��ʵ�����к��ȵ���ֵƫС��c�������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�d��ȡH2SO4��Һ�����ʱ���Ӷ������ᵼ��������H2SO4���ƫ�ų�������ƫ�ߣ�e�����������ɢʧ������ͨ�����β������������裬
���
�⣺��1����ϡ��Һ�У��������кͷ�Ӧ����1 molˮʱ�ķ�Ӧ�Ƚ����к��ȣ�����Ҫ�㣺������1 molˮΪ�������к��ȸ��������ϡ������ϡ�ռ���Һ�к��ȵ��Ȼ�ѧ����ʽΪ��
H2SO4��aq��+NaOH��aq��=
Na2SO4��aq��+H2O��l����H=-57.3 kJ/mol��
�ʴ�Ϊ��
H2SO4��aq��+NaOH��aq��=
Na2SO4��aq��+H2O��l����H=-57.3 kJ/mol��
��2�������������Ϊ98%���ܶ�Ϊ1.8g/cm3��Ũ���ᣬ�����ʵ���Ũ��c=
mol/L=18.0mol/L
����Ҫ����Ũ��������ΪVmL������ϡ�Ͷ��ɣ���18.0 mol/L��VmL=0.25 mol/L��245mL�����V=3.5mL���ʴ�Ϊ��3.5mL
������������Һ��������ֱ��Ǽ��㡢��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ����ݸ���������Ҫʹ�õ�������bcdfg���ʴ�Ϊ��bcdfg��
��ٸ���������װ��ͼ����֪k������Ϊ�����β����������
ʵ��װ�ñ��¡�����Ч������ã�����Ӱ��ʵ����������װ��ȱ��Ӳֽ�壬�ʴ�Ϊ��δ��Ӳֽ�壨�����ϰ壩��ס�ձ���
���ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ�����²���к�����ֵ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��0.55mol/L��NaOH��Һ50mL��0.25mol/L��������Һ50mL�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol=0.025mol����Һ������Ϊ��100ml��1g/ml=100g���¶ȱ仯��ֵ��TΪ3.2�棬������0.025molˮ�ų�������ΪQ=m?c?��T=100g��4.18J/��g?�棩��3.2��=1337.6J����1.337kJ������ʵ���õ��к��ȡ�H=-
=-53.5 kJ/mol��
�ʴ�Ϊ��-53.5 kJ/mol��
��a������Ͳ��ȡ��Һǰ��Ҫ��ϴ�����������ȡ����Һ���ϡ�ͣ������к���������a���������⣮
b������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС����b�������⣮
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��������������ɢʧ�������к���������c�������⣮
d����ȡH2SO4��Һ�����ʱ���Ӷ������ᵼ��������H2SO4���ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��d�������⣮
e���ҿ�ӲֽƬ�ò��������裬�ᵼ������ɢʧ��Ӱ��ⶨ�������e�������⣮
�ʴ�Ϊbcde��
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��
| 1 |
| 2 |
| 1 |
| 2 |
��2�������������Ϊ98%���ܶ�Ϊ1.8g/cm3��Ũ���ᣬ�����ʵ���Ũ��c=
| 1000��1.8��98% |
| 98 |
����Ҫ����Ũ��������ΪVmL������ϡ�Ͷ��ɣ���18.0 mol/L��VmL=0.25 mol/L��245mL�����V=3.5mL���ʴ�Ϊ��3.5mL
������������Һ��������ֱ��Ǽ��㡢��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ����ݸ���������Ҫʹ�õ�������bcdfg���ʴ�Ϊ��bcdfg��
��ٸ���������װ��ͼ����֪k������Ϊ�����β����������
ʵ��װ�ñ��¡�����Ч������ã�����Ӱ��ʵ����������װ��ȱ��Ӳֽ�壬�ʴ�Ϊ��δ��Ӳֽ�壨�����ϰ壩��ס�ձ���
���ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ�����²���к�����ֵ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��0.55mol/L��NaOH��Һ50mL��0.25mol/L��������Һ50mL�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol=0.025mol����Һ������Ϊ��100ml��1g/ml=100g���¶ȱ仯��ֵ��TΪ3.2�棬������0.025molˮ�ų�������ΪQ=m?c?��T=100g��4.18J/��g?�棩��3.2��=1337.6J����1.337kJ������ʵ���õ��к��ȡ�H=-
| 1.337KJ |
| 0.025mol |
�ʴ�Ϊ��-53.5 kJ/mol��
��a������Ͳ��ȡ��Һǰ��Ҫ��ϴ�����������ȡ����Һ���ϡ�ͣ������к���������a���������⣮
b������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС����b�������⣮
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��������������ɢʧ�������к���������c�������⣮
d����ȡH2SO4��Һ�����ʱ���Ӷ������ᵼ��������H2SO4���ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��d�������⣮
e���ҿ�ӲֽƬ�ò��������裬�ᵼ������ɢʧ��Ӱ��ⶨ�������e�������⣮
�ʴ�Ϊbcde��
�����������ۺϿ������к��ȵIJⶨ���������Լ��к��ȵ��Ȼ�ѧ����ʽ����д���ۺ��Խ�ǿ���ѶȽϴ�

��ϰ��ϵ�д�
 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�
�����Ŀ
��Ӧ3Fe��S��+4H2O��g��=Fe3O4��s��+4H2��g������һ�ɱ���ݻ����ܱ������н��У�����˵����ȷ���ǣ�������
| A������Fe������������Ӧ�������� |
| B���������������Сһ�룬������Ӧ���������淴Ӧ���ʼ�С |
| C������������䣬����ˮ����ʹ��ϵѹǿ����������Ӧ���������淴Ӧ���ʼ�С |
| D������ѹǿ���䣬����N2ʹ�������������������Ӧ���ʼ�С���淴Ӧ����Ҳ��С |
�л���Ļ�ѧ������Ҫ��������ž�����ƻ������һ���л��ᣬ�ṹ��ʽΪ��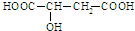 �������й�ƻ�����˵������ȷ���ǣ�������
�������й�ƻ�����˵������ȷ���ǣ�������
 �������й�ƻ�����˵������ȷ���ǣ�������
�������й�ƻ�����˵������ȷ���ǣ�������| A��1molƻ�������������Ʒ�Ӧ����������3g |
| B��1molƻ��������������������Һ��Ӧ������3mol�������� |
| C��1molƻ��������������̼��������Һ��Ӧ�����������44.8L�Ķ�����̼���� |
| D��ƻ������һ�������¼�������ᷴӦ���������Ҵ���Ӧ��Ҳ������������������Ӧ |
�����й�����Ũ�ȹ�ϵ�������У���ȷ���ǣ�������
| A��25��ʱ����0.1mol?L-1 NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ�c��Na+����c��NH4+����c��SO42-����c��OH-��=c��H+�� |
| B��25��ʱ��NaB��Һ��pH=8��c��Na+��+c��B-��=9.9��10-7mol?L-1 |
| C��0.1mol?L-1��NaHCO3��Һ�У�c��OH-��+c��CO32-��=c��H+��+c��H2CO3�� |
| D��ͬ���£�pH��ͬʱ����Һ���ʵ���Ũ�ȣ�c��CH3COONa����c��NaHCO3����c��C6H5ONa����c��Na2CO3�� |
���и���Ԫ�ذ��縺�Դ�С������ȷ���ǣ�������
| A��F��N��O |
| B��O��Cl��F |
| C��As��P��H |
| D��Cl��S��As |
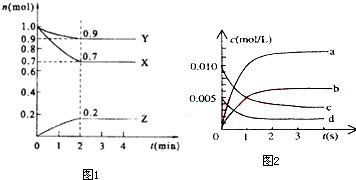
 ����t��ʱ��ijNaOHϡ��Һ�У�c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12����
����t��ʱ��ijNaOHϡ��Һ�У�c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12����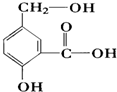 ��ij�л���A�ķ�����ṹ��ʽ��ͼ����ش�
��ij�л���A�ķ�����ṹ��ʽ��ͼ����ش�