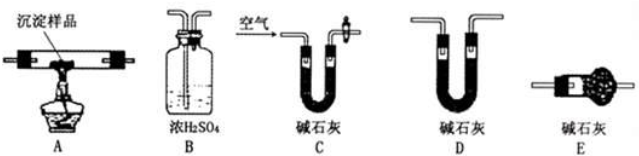
��Ŀ����
ij�����о���ѧϰС�����ô��Σ������������ʣ�������ɳ��CaCl2��MgCl2��Na2SO4�����ᴿ������0.4mol/L 450mL NaCl��Һ��������Ʋ�ʵʩ������ʵ�飬�������ѧ����֪ʶ�ش��������⣺
��1��ȡһ�����Ĵ��������ձ��м�ˮ�ܽ⣬���ӵ�ˮ��ӦΪ�� ������ѡ�
A�����ˮ��ɽ�ϡ����Һ B������������ˮ�����ܽ�Ĺ����ܽ�Ϊֹ
��2������1�������ƵĴ�������Һ���˺�ȡ��Һ��������ij����Լ�����������Լ����Դ�����������Һ��ѡ��Na2CO3��Һ ��KOH��Һ ��AgNO3��Һ ��NaOH��Һ ��NaHCO3��Һ ��BaCl2��Һ������ȷ���Լ��ͼ����˳��ӦΪ�� ������ѡ���ѡ����ѡ����ѡ�����÷֣�
A���٢ڢ�B���ڢޢ�C���ܢޢ�D���٢ܢ�E���ޢ٢�F���ޢڢ�
��3�����ã�2�������õ�NaCl�������������Һ����������ƽ�ϳ��� g NaCl���壮���ƹ�������Ҫʹ�õIJ��������У��ձ����������� �� ��
��4�����ƽ�����ͬѧ�Ƕ�ʵ���г��ֵ�ijЩ������������������Ƶ���ҺŨ�ȵ�Ӱ������˷����������д�������ᵼ������������Һ��NaCl��Ũ�����0.4mol/L��ƫ���ǣ����ƫ����ƫС��������Ӱ�족��
����������2��������û��ʹ�����ᴦ����Һ������� ��
��û�ж��ձ��Ͳ���������ϴ�ӣ������ ��
�۶���ʱͨ������ǰ�ظ��Ӱ�Һ�棬����� ��
�����µߵ�ҡ�Ⱥ�Һ����ڿ̶��ߣ�δ��ʱ��ˮ���̶��ߣ������ ��
��1��ȡһ�����Ĵ��������ձ��м�ˮ�ܽ⣬���ӵ�ˮ��ӦΪ��
A�����ˮ��ɽ�ϡ����Һ B������������ˮ�����ܽ�Ĺ����ܽ�Ϊֹ
��2������1�������ƵĴ�������Һ���˺�ȡ��Һ��������ij����Լ�����������Լ����Դ�����������Һ��ѡ��Na2CO3��Һ ��KOH��Һ ��AgNO3��Һ ��NaOH��Һ ��NaHCO3��Һ ��BaCl2��Һ������ȷ���Լ��ͼ����˳��ӦΪ��
A���٢ڢ�B���ڢޢ�C���ܢޢ�D���٢ܢ�E���ޢ٢�F���ޢڢ�
��3�����ã�2�������õ�NaCl�������������Һ����������ƽ�ϳ���
��4�����ƽ�����ͬѧ�Ƕ�ʵ���г��ֵ�ijЩ������������������Ƶ���ҺŨ�ȵ�Ӱ������˷����������д�������ᵼ������������Һ��NaCl��Ũ�����0.4mol/L��ƫ���ǣ����ƫ����ƫС��������Ӱ�족��
����������2��������û��ʹ�����ᴦ����Һ�������
��û�ж��ձ��Ͳ���������ϴ�ӣ������
�۶���ʱͨ������ǰ�ظ��Ӱ�Һ�棬�����
�����µߵ�ҡ�Ⱥ�Һ����ڿ̶��ߣ�δ��ʱ��ˮ���̶��ߣ������
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺ʵ����
��������1���ܽ����ʱ�������ˮ��ʹ����ǡ���ܽ⼴�ɣ�
��2�����ݺ��е�������CaCl2��MgCl2��Na2SO4�����ݼ��ܳ�ȥ���ʻ��������������ʵ�ԭ��ѡ������ʵij����Լ��ǣ���Na2CO3��Һ ��NaOH��Һ ��BaCl2��Һ��Ȼ����ݢ�Na2CO3��Һ�������Dz���Ҫ��ȥCaCl2����Ҫ��ȥ������BaCl2���ʢ�Na2CO3��Һ�ļ���һ��Ҫ�ڢ�BaCl2��Һ֮�ó������Լ���˳��
��3������n=cV��m=nM�����㣻�������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��4������c=
��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��2�����ݺ��е�������CaCl2��MgCl2��Na2SO4�����ݼ��ܳ�ȥ���ʻ��������������ʵ�ԭ��ѡ������ʵij����Լ��ǣ���Na2CO3��Һ ��NaOH��Һ ��BaCl2��Һ��Ȼ����ݢ�Na2CO3��Һ�������Dz���Ҫ��ȥCaCl2����Ҫ��ȥ������BaCl2���ʢ�Na2CO3��Һ�ļ���һ��Ҫ�ڢ�BaCl2��Һ֮�ó������Լ���˳��
��3������n=cV��m=nM�����㣻�������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��4������c=
| n |
| V |
���
�⣺��1���ܽ����ʱ������������ˮ��ʹ����ǡ���ܽ⼴�ɣ���ѡB��
��2����ȥʳ���е�CaCl2�â�Na2CO3��Һ����ȥMgCl2�â�NaOH��Һ����ȥNa2SO4�â�BaCl2��Һ������Na2CO3��Һ�������Dz���Ҫ��ȥCaCl2����Ҫ��ȥ������BaCl2���ʢ�Na2CO3��Һ�ļ���һ��Ҫ�ڢ�BaCl2��Һ֮����ȷ���Լ��ͼ����˳��ӦΪ���ޢ٢ܻ�ޢܢٻ�ܢޢ٣���ѡCE��
��3��������450mL����ƿ��������500mL������ƿ�����Ƴ�500ML����Һ��������Ȼ��Ƶ����ʵ���n=cV=0.5L��0.4mol/L=0.2mol������m=nM=0.2mol��58.5g/mol=11.7g��
���������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��11.7��500mL����ƿ����ͷ�ιܣ�
��4������������2��������û��ʹ�����ᴦ����Һ���ᵼ����Һ����δ��Ӧ����Na2CO3��NaOH����ᵼ������������Һ��NaCl��Ũ�����0.4mol/L��ƫС���ʴ�Ϊ��ƫС��
��û�ж��ձ��Ͳ���������ϴ�ӣ���ᵼ��������ʧ��������Һ��Ũ��ƫС���ʴ�Ϊ��ƫС��
�۶���ʱͨ������ǰ�ظ��Ӱ�Һ�棬�ᵼ����Һ���ƫС����Ũ��ƫ�ʴ�Ϊ��ƫ��
�����µߵ�ҡ�Ⱥ�Һ����ڿ̶����������ģ�δ��ˮ���̶�������ȷ�ģ��ʶԽ����Ӱ�죬�ʴ��⣺��Ӱ�죮
��2����ȥʳ���е�CaCl2�â�Na2CO3��Һ����ȥMgCl2�â�NaOH��Һ����ȥNa2SO4�â�BaCl2��Һ������Na2CO3��Һ�������Dz���Ҫ��ȥCaCl2����Ҫ��ȥ������BaCl2���ʢ�Na2CO3��Һ�ļ���һ��Ҫ�ڢ�BaCl2��Һ֮����ȷ���Լ��ͼ����˳��ӦΪ���ޢ٢ܻ�ޢܢٻ�ܢޢ٣���ѡCE��
��3��������450mL����ƿ��������500mL������ƿ�����Ƴ�500ML����Һ��������Ȼ��Ƶ����ʵ���n=cV=0.5L��0.4mol/L=0.2mol������m=nM=0.2mol��58.5g/mol=11.7g��
���������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��11.7��500mL����ƿ����ͷ�ιܣ�
��4������������2��������û��ʹ�����ᴦ����Һ���ᵼ����Һ����δ��Ӧ����Na2CO3��NaOH����ᵼ������������Һ��NaCl��Ũ�����0.4mol/L��ƫС���ʴ�Ϊ��ƫС��
��û�ж��ձ��Ͳ���������ϴ�ӣ���ᵼ��������ʧ��������Һ��Ũ��ƫС���ʴ�Ϊ��ƫС��
�۶���ʱͨ������ǰ�ظ��Ӱ�Һ�棬�ᵼ����Һ���ƫС����Ũ��ƫ�ʴ�Ϊ��ƫ��
�����µߵ�ҡ�Ⱥ�Һ����ڿ̶����������ģ�δ��ˮ���̶�������ȷ�ģ��ʶԽ����Ӱ�죬�ʴ��⣺��Ӱ�죮
���������⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
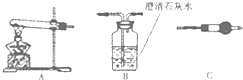 ij�о���ѧϰС�齫һ��Ũ��Na2CO3��Һ����CuSO4��Һ�еõ�������
ij�о���ѧϰС�齫һ��Ũ��Na2CO3��Һ����CuSO4��Һ�еõ�������