��Ŀ����
 ij�о���ѧϰС�齫һ��Ũ��Na2CO3��Һ����CuSO4��Һ�еõ�������
ij�о���ѧϰС�齫һ��Ũ��Na2CO3��Һ����CuSO4��Һ�еõ���������ͬѧ��Ϊ���߷�Ӧֻ����CuSO3һ�ֳ�����
��ͬѧ��Ϊ������Һ����ٽ�ˮ�⣬���������Cu��OH��2һ�ֳ�����
��ͬѧ��Ϊͬʱ����CuCO3��Cu��OH��2���ֳ���������֪��CuCO3��Cu��OH��2�������ᾧˮ��
I
��1��д��Na2CO3��Һˮ������ӷ���ʽ
��2����̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ��
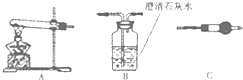
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ������ж���̽��������ijɷ֣�
��1����װ������˳��Ϊ
��2��װ��C��װ���Լ���������
��3����֤������������CuCO3��ʵ��������
����CuCO3��Cu��OH��2���߶��У���ͨ������װ�õ����ӣ����ж����������ⶨ����ɣ�
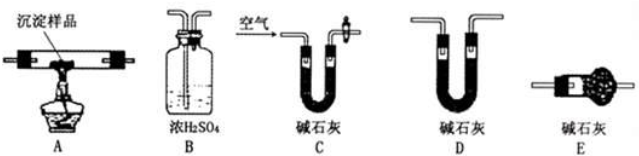
��1��װ��c�м�ʯ�ҵ�������
��2��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ�˴������Ĺ��������������÷ֱ��ǣ�
��3����������Ʒ������Ϊm g��װ��B����������n g���������CuCO3������������Ϊ
���㣺����ʵ�鷽�������,���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı���
ר�⣺ʵ�������
��������1��̼���Ʒֲ�ˮ�⣻
��2���Ƚ���������Һ���룬��ȡ���˷�����Ȼ��ϴ�ӳ������ŵ����ʣ��ٽ��и���ɵýϴ����ij�����
�����ü��ȵķ������飬������ͭ��̼��ͭ���ȷֽ�õ�����ͭ��ˮ�Ͷ�����̼������������ͭ������ˮ����ͭ���飬����̼��ͭ���ó����ʯ��ˮ��������Ķ�����̼������ʯ��ˮ�����˵������CuCO3��
��1��ʵ�鿪ʼʱװ�õĿ����л���ˮ�����Ͷ�����̼�������ų�������װ�����ջ�Լ����������ϴ�����ʿ�ʼʱ���ó�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ų���
��2��ʵ��ǰװ���к��к�ˮ������CO2�Ŀ���������Ҫ�Ƚ����ų���ʵ��ʱ������ͭ��̼��ͭ���ȷֽ����ڷ�Ӧװ���в���������̼��ˮ������ͨ��ͨ������ij�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ϳ�������װ����ȫ���գ���ֹӰ��ⶨ�����
��3��װ��B����������n�ˣ�˵���ֽ�����ngˮ������ˮ���������������������ͭ������������������ȥ������ͭ����������̼��ͭ�����������������������Ķ�����㣮
��2���Ƚ���������Һ���룬��ȡ���˷�����Ȼ��ϴ�ӳ������ŵ����ʣ��ٽ��и���ɵýϴ����ij�����
�����ü��ȵķ������飬������ͭ��̼��ͭ���ȷֽ�õ�����ͭ��ˮ�Ͷ�����̼������������ͭ������ˮ����ͭ���飬����̼��ͭ���ó����ʯ��ˮ��������Ķ�����̼������ʯ��ˮ�����˵������CuCO3��
��1��ʵ�鿪ʼʱװ�õĿ����л���ˮ�����Ͷ�����̼�������ų�������װ�����ջ�Լ����������ϴ�����ʿ�ʼʱ���ó�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ų���
��2��ʵ��ǰװ���к��к�ˮ������CO2�Ŀ���������Ҫ�Ƚ����ų���ʵ��ʱ������ͭ��̼��ͭ���ȷֽ����ڷ�Ӧװ���в���������̼��ˮ������ͨ��ͨ������ij�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ϳ�������װ����ȫ���գ���ֹӰ��ⶨ�����
��3��װ��B����������n�ˣ�˵���ֽ�����ngˮ������ˮ���������������������ͭ������������������ȥ������ͭ����������̼��ͭ�����������������������Ķ�����㣮
���
�⣺��1��̼���Ʒֲ�ˮ�⣬ˮ�����ӷ���ʽΪ��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-��
��2���Ƚ���������Һ���룬��ȡ���˷�����Ȼ��ϴ�ӳ������ŵ����ʣ��ٽ��и���ɵýϴ����ij�����
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
��1�����ü��ȵķ������飬������ͭ��̼��ͭ���ȷֽ�õ�����ͭ��ˮ�Ͷ�����̼������������ͭ������ˮ����ͭ���������ˮ������̼��ͭ���ó����ʯ��ˮ��������Ķ�����̼����ˮһ�㶼�ȼ�����Ϊ����Ҫͨ����Һ�����ˮ�������ʴ�Ϊ��A��C��B��
��2�����ݣ�1���ķ�����װ��C��װ���Լ�����������ˮ����ͭ���ʴ�Ϊ����ˮ����ͭ��
��3���ó����ʯ��ˮ�����Ƿ����������̼��װ��B�г���ʯ��ˮ����ǣ�˵�����ɶ�����̼����˵������CuCO3��
�ʴ�Ϊ��װ��B�г���ʯ��ˮ����ǣ�
��1��ʵ�鿪ʼʱװ�õĿ����л���ˮ�����Ͷ�����̼�������ų�������װ�����ջ�Լ����������ϴ�����ʿ�ʼʱ���ó�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ų�����װ��C�м�ʯ�ҵ����������տ����е�ˮ������CO2���ʴ�Ϊ�����տ����е�ˮ������CO2��
��2��ʵ��ǰװ���к��к�ˮ������CO2�Ŀ���������Ҫ�Ƚ����ų���ʵ��ʱ������ͭ��̼��ͭ���ȷֽ����ڷ�Ӧװ���в���������̼��ˮ������ͨ��ͨ������ij�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ϳ�������װ����ȫ���գ���ֹӰ��ⶨ�����
�ʴ�Ϊ��ʵ��ǰ��Ϊ�˽�װ����ԭ�к�ˮ������CO2�Ŀ����ų���ʵ�����Ϊ�˽�װ����������ˮ������CO2�μӷ�Ӧ��
��3��װ��B����������n�ˣ�˵���ֽ�����ngˮ��ˮ�����ʵ���Ϊ
mol��������Ԫ���غ��֪������ͭ�����ʵ���Ϊ
mol����������ͭ������Ϊ
mol��98g/mol=
g��������CuCO3����������Ϊ
��100%=��1-
����100%��
�ʴ�Ϊ����1-
����100%��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-��
��2���Ƚ���������Һ���룬��ȡ���˷�����Ȼ��ϴ�ӳ������ŵ����ʣ��ٽ��и���ɵýϴ����ij�����
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
��1�����ü��ȵķ������飬������ͭ��̼��ͭ���ȷֽ�õ�����ͭ��ˮ�Ͷ�����̼������������ͭ������ˮ����ͭ���������ˮ������̼��ͭ���ó����ʯ��ˮ��������Ķ�����̼����ˮһ�㶼�ȼ�����Ϊ����Ҫͨ����Һ�����ˮ�������ʴ�Ϊ��A��C��B��
��2�����ݣ�1���ķ�����װ��C��װ���Լ�����������ˮ����ͭ���ʴ�Ϊ����ˮ����ͭ��
��3���ó����ʯ��ˮ�����Ƿ����������̼��װ��B�г���ʯ��ˮ����ǣ�˵�����ɶ�����̼����˵������CuCO3��
�ʴ�Ϊ��װ��B�г���ʯ��ˮ����ǣ�
��1��ʵ�鿪ʼʱװ�õĿ����л���ˮ�����Ͷ�����̼�������ų�������װ�����ջ�Լ����������ϴ�����ʿ�ʼʱ���ó�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ų�����װ��C�м�ʯ�ҵ����������տ����е�ˮ������CO2���ʴ�Ϊ�����տ����е�ˮ������CO2��
��2��ʵ��ǰװ���к��к�ˮ������CO2�Ŀ���������Ҫ�Ƚ����ų���ʵ��ʱ������ͭ��̼��ͭ���ȷֽ����ڷ�Ӧװ���в���������̼��ˮ������ͨ��ͨ������ij�ȥˮ�Ͷ�����̼�Ŀ�����װ���е�ˮ�����Ͷ�����̼�ϳ�������װ����ȫ���գ���ֹӰ��ⶨ�����
�ʴ�Ϊ��ʵ��ǰ��Ϊ�˽�װ����ԭ�к�ˮ������CO2�Ŀ����ų���ʵ�����Ϊ�˽�װ����������ˮ������CO2�μӷ�Ӧ��
��3��װ��B����������n�ˣ�˵���ֽ�����ngˮ��ˮ�����ʵ���Ϊ
| n |
| 18 |
| n |
| 18 |
| n |
| 18 |
| 49n |
| 9 |
m-
| ||
| m |
| 49n |
| 9m |
�ʴ�Ϊ����1-
| 49n |
| 9m |
�����������ʵ�鷽�������װ�õ����⡢ʵ�������������ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���� ����Ĺؼ����Ƕ�֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ������֪ʶ�������⡢��������������

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
ʵ����Ҫ��98%���ܶ�Ϊ1.84g?cm-3������������3.68mol/L������500mL��������3.68mol/L�����ᣬ������������ȷ�����в�����ʹ����������ҺŨ��ƫ�͵��ǣ�������
| A����ϡ�͵�����ת��������ƿ��δϴ���ձ��Ͳ����� |
| B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� |
| C���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ���������ƿ�̶��ߣ���ʱ�����ý�ͷ�ιܽ�ƿ�ڶ���Һ��������ʹ��Һ��Һ����̶������� |
| D���ý�ͷ�ι�������ƿ�м���ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶������� |
���и���ʵ������Һ���ȱ���ǵ��ǣ�������
| A��0.1mol/LNa2S2O3��H2SO4��5mL����ˮ5mL����Ӧ�¶�10�� |
| B��0.1mol/LNa2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�10�� |
| C��0.1mol/LNa2S2O3��H2SO4��5mL����ˮ5mL����Ӧ�¶�30�� |
| D��0.1mol/LNa2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�30�� |
�±�Ϊ����һ�ȴ���Ľṹ��ʽ��һЩ�������ݣ����жԱ������������ݵķ������ɴ�����ǣ�������
| ��� | �ṹ��ʽ | �е�/�� | ����ܶ� |
| �� | CH3Cl | -24.2 | 0.915 9 |
| �� | CH3CH2Cl | 12.3 | 0.897 8 |
| �� | CH3CH2CH2Cl | 46.6 | 0.890 9 |
| �� | CH3CHClCH3 | 35.7 | 0.861 7 |
| �� | CH3CH2CH2CH2Cl | 78.44 | 0.886 2 |
| �� | CH3CH2CHClCH3 | 68.2 | 0.873 2 |
| �� | ��CH3��3CCl | 52 | 0.842 0 |
| A�����ʢ٢ڢۢݿɻ����Ϊͬϵ�� |
| B��һ�ȴ���ͬ���칹��ķе�����֧������������� |
| C��һ�ȴ���ķе�����̼����������������� |
| D��һ�ȴ��������ܶ�����̼������������ڼ�С |

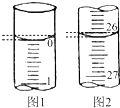 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�