��Ŀ����
���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����4.1g�������������������ʵ���Ʒ�����250mL������Һ������ʱ��Ʒ�ɷ��� ��������ĸ�� �ϳ���
��A��С�ձ� ��B���ྻֽƬ ��C��ֱ�ӷ���������
��2���ζ�ʱ��һ�㲻��ѡ�� ��������ĸ����ָʾ����
��A������ ��B��ʯ�� ��C����̪
��3�������йصζ�������˳����ȷ����
�ټ��ζ����Ƿ�©ˮ ��������ˮϴ�Ӳ������� ���ñ���Һ��ϴʢ����Һ�ĵζ��ܣ��ô���Һ��ϴʢ����Һ�ĵζ��� ��װ����Һ�ʹ���Һ������Һ�棨��¼�������� ��ȡһ������Ĵ���Һ����ƿ�� �ζ�����
��4����0.2010mol?L-1������ζ������ռ���Һ���ζ�����ȷ���������� ��
��5����������ѡ���ָʾ������ȷ�жϵζ��յ��������
��6�������±����ݣ����㱻���ռ�Ũ��Ϊ ���ռ���Ʒ�Ĵ���Ϊ ��
��7������ʵ�������Եζ���������ĺ�������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ���� ��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ���� ��
��1��ȷ����4.1g�������������������ʵ���Ʒ�����250mL������Һ������ʱ��Ʒ�ɷ���
��A��С�ձ� ��B���ྻֽƬ ��C��ֱ�ӷ���������
��2���ζ�ʱ��һ�㲻��ѡ��
��A������ ��B��ʯ�� ��C����̪
��3�������йصζ�������˳����ȷ����
�ټ��ζ����Ƿ�©ˮ ��������ˮϴ�Ӳ������� ���ñ���Һ��ϴʢ����Һ�ĵζ��ܣ��ô���Һ��ϴʢ����Һ�ĵζ��� ��װ����Һ�ʹ���Һ������Һ�棨��¼�������� ��ȡһ������Ĵ���Һ����ƿ�� �ζ�����
��4����0.2010mol?L-1������ζ������ռ���Һ���ζ�����ȷ����������
��5����������ѡ���ָʾ������ȷ�жϵζ��յ��������
��6�������±����ݣ����㱻���ռ�Ũ��Ϊ
| �ζ����� | ������Һ�����ml�� | �����������ml�� | |
| �ζ�ǰ�̶ȣ�ml�� | �ζ���̶ȣ�ml�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 0.20 | 20.80 |
| ������ | 10.00 | 4.10 | 24.00 |
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����
���㣺�к͵ζ�
ר�⣺����ƽ������Һ��pHר��
��������1���׳����ҩƷ��������ڲ��������ϣ���С�ձ���������������
��2������к͵ζ�ʱ�����÷�̪�������ָʾ����ʯ���ɫ��Χ�����������ԣ���һ�㲻��ʯ����ָʾ����
��3���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ���ζ��Ȳ�����
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯���Ա�ȷ�ж��յ�ĵ��
��5����̪�ڼ�����Һ���Ժ�ɫ���ﵽ�յ�ʱ���к�ɫ����ɫ��
��6���ȷ������ݵĿ��Ŷȣ���ȥ���̫������ݣ��ٸ���C�����⣩�T
�����C�����⣩��V�����������ε�ƽ��ֵ��Ȼ�����m=CVM����250mL�ռ���Ʒ���������Ƶ��������ٸ�������������ʽ�����������Ƶ�����������
��7����C�����⣩�T
������
��2������к͵ζ�ʱ�����÷�̪�������ָʾ����ʯ���ɫ��Χ�����������ԣ���һ�㲻��ʯ����ָʾ����
��3���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ���ζ��Ȳ�����
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯���Ա�ȷ�ж��յ�ĵ��
��5����̪�ڼ�����Һ���Ժ�ɫ���ﵽ�յ�ʱ���к�ɫ����ɫ��
��6���ȷ������ݵĿ��Ŷȣ���ȥ���̫������ݣ��ٸ���C�����⣩�T
| c(��)��V(��) |
| V(����) |
��7����C�����⣩�T
| c(��)��V(��) |
| V(����) |
���
�⣺��1���׳����ҩƷ��������ڲ��������ϣ���С�ձ����������������ռ��׳��⣬����Ӧ����С�ձ��г�������ѡ��A��
��2������к͵ζ�ʱ�����÷�̪�������ָʾ����ʯ���ɫ��Χ�����������ԣ���һ�㲻��ʯ����ָʾ������ѡ��B��
��3���⣺�к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ���ζ���˳������������˳��Ϊ���٢ڢۢܢݢޣ��ʴ�Ϊ���٢ڢۢܢݢޣ�
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯���Ա�ȷ�ж��յ�ĵ�����Ա�ȷ�ж��յ�ĵ���ʴ�Ϊ��������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
��5����̪�ڼ�����Һ���Ժ�ɫ�����������һ�����ᣬ��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ���Ұ���Ӳ��ָ���ɫ��֤���ﵽ�ζ��յ㣬�ʴ�Ϊ�����������һ�����ᣬ��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ���Ұ���Ӳ��ָ���ɫ��
��6���ڶ���ʵ�����̫����ȥ��V�������T[��20.50-0.40��+��24.00-4.10��]mL��2�T20.00mL��
C�����⣩�T
=
=0.4020mol/L��
��m�TCVM�T0.4020mol?L-1��0.25L��40g/mol�T4.02g
�أ�NaOH���T
��100%�T98%��
�ʴ�Ϊ��0.4020mol/L��98%��
��7���ٶ������ζ�ǰ���ӣ��ζ���ƽ�ӣ����±�Һ�����ƫС������C�����⣩�T
������֪C�����⣩ƫС���ʴ�Ϊ��ƫ�ͣ�
���ô���Һ��ϴ��ƿ������Һ�����ʵ���ƫ���±�Һ�����ƫ����C�����⣩�T
������֪C�����⣩ƫ�ʴ�Ϊ��ƫ�ߣ�
��2������к͵ζ�ʱ�����÷�̪�������ָʾ����ʯ���ɫ��Χ�����������ԣ���һ�㲻��ʯ����ָʾ������ѡ��B��
��3���⣺�к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ���ζ���˳������������˳��Ϊ���٢ڢۢܢݢޣ��ʴ�Ϊ���٢ڢۢܢݢޣ�
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯���Ա�ȷ�ж��յ�ĵ�����Ա�ȷ�ж��յ�ĵ���ʴ�Ϊ��������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
��5����̪�ڼ�����Һ���Ժ�ɫ�����������һ�����ᣬ��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ���Ұ���Ӳ��ָ���ɫ��֤���ﵽ�ζ��յ㣬�ʴ�Ϊ�����������һ�����ᣬ��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ���Ұ���Ӳ��ָ���ɫ��
��6���ڶ���ʵ�����̫����ȥ��V�������T[��20.50-0.40��+��24.00-4.10��]mL��2�T20.00mL��
C�����⣩�T
| c(��)��V(��) |
| V(����) |
| 0.2010mol/L��0.02000L |
| 0.0100L |
��m�TCVM�T0.4020mol?L-1��0.25L��40g/mol�T4.02g
�أ�NaOH���T
| 4.02g |
| 4.1g |
�ʴ�Ϊ��0.4020mol/L��98%��
��7���ٶ������ζ�ǰ���ӣ��ζ���ƽ�ӣ����±�Һ�����ƫС������C�����⣩�T
| c(��)��V(��) |
| V(����) |
���ô���Һ��ϴ��ƿ������Һ�����ʵ���ƫ���±�Һ�����ƫ����C�����⣩�T
| c(��)��V(��) |
| V(����) |
������������Ҫ�������к͵ζ��������������Լ��ռ�Ĵ��ȵļ��㣬�Ѷ��еȣ�ע��֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
������ˮ������ǣ�������
| A�����顢��ϩ |
| B�����顢���� |
| C��NaCl��Һ��KCl��Һ |
| D��MgSO4��Һ��Mg��NO3��2��Һ |

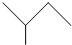
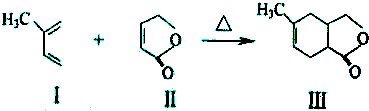
 ��CH��CH����Ȳ��Ҳ�ܷ���Diels-Alder��Ӧ����д���÷�Ӧ����Ľṹ��ʽ
��CH��CH����Ȳ��Ҳ�ܷ���Diels-Alder��Ӧ����д���÷�Ӧ����Ľṹ��ʽ