��Ŀ����
ij���᳧�����¼��ַ�������SO2β����
��1������̿��ԭ����Ӧԭ�������º��ݣ�2C ��s��+2SO2��g���TS2��g��+2CO2��g������Ӧ���е���ͬʱ���ø����ʵ�Ũ����ͼ1��

�ٵ�һ�γ���ƽ���ʱ���ǵ� min��
��0��20min��Ӧ���ʱ�ʾΪV��SO2��= ��
��30minʱ���ı�ijһ����ƽ�ⷢ���ƶ�����ı���������п����� ��40minʱ��ƽ�ⳣ��ֵΪ ��
��2�������������շ�
��Na2SO3��Һ����SO2�����ӷ���ʽΪ ��
�ڳ����£���������pH=6ʱ������Һ���������Ũ�ȹ�ϵһ����ȷ���� ������ţ�
a��c��Na+��+c��H+����c��SO32-��+c��HSO3-��+c��OH-��
b��c��Na+��=c��SO32-��+c��HSO3-��+C��H2SO3��
c��c��Na-����c��SO32-����c��OHһ����c��H+��
d��ˮ�����c��OHһ��=l��l0-8 mol/L��
��3���绯ѧ������
��ͼ2��ʾ��Pt��1���缫�ķ�ӦʽΪ �����������£���P��2���缫�ų���S2O42-��Һ����NO2��ʹ��ת��ΪN2��ͬʱ��SO32-���ɣ�������ת�Ƶ���6mol���������ϴ���NO2���� mol��
��1������̿��ԭ����Ӧԭ�������º��ݣ�2C ��s��+2SO2��g���TS2��g��+2CO2��g������Ӧ���е���ͬʱ���ø����ʵ�Ũ����ͼ1��

�ٵ�һ�γ���ƽ���ʱ���ǵ�
��0��20min��Ӧ���ʱ�ʾΪV��SO2��=
��30minʱ���ı�ijһ����ƽ�ⷢ���ƶ�����ı���������п�����
��2�������������շ�
��Na2SO3��Һ����SO2�����ӷ���ʽΪ
�ڳ����£���������pH=6ʱ������Һ���������Ũ�ȹ�ϵһ����ȷ����
a��c��Na+��+c��H+����c��SO32-��+c��HSO3-��+c��OH-��
b��c��Na+��=c��SO32-��+c��HSO3-��+C��H2SO3��
c��c��Na-����c��SO32-����c��OHһ����c��H+��
d��ˮ�����c��OHһ��=l��l0-8 mol/L��
��3���绯ѧ������
��ͼ2��ʾ��Pt��1���缫�ķ�ӦʽΪ
���㣺���ʵ�����Ũ����ʱ��ı仯����,��ѧƽ��ļ���,���ԭ��,����Ũ�ȴ�С�ıȽ�
ר�⣺��ѧƽ��ר��,�绯ѧר��
��������1���ٸ����Ũ�Ȳ��䣬��Ӧ�ﵽƽ��״̬��
�ڸ��ݷ�Ӧ����v=
���㣻
��30minʱ��CO2Ũ�ȼ�С����������Ũ�Ȳ��䣬�ı�������Ǽ�СCO2��Ũ�ȣ�����ƽ�ⳣ��K=
���㣻
��2����Na2SO3��Һ����SO2����NaHSO3��
�ڳ����£���������pH=6ʱ����Һ�����ԣ�
��3��Pt��1���缫����SO2������Ũ������SO2��֪ʧ���ӣ���������ÿmolNO2�õ���4mol��
�ڸ��ݷ�Ӧ����v=
| ��c |
| ��t |
��30minʱ��CO2Ũ�ȼ�С����������Ũ�Ȳ��䣬�ı�������Ǽ�СCO2��Ũ�ȣ�����ƽ�ⳣ��K=
| c2(CO2)?c(S2) |
| c2(SO2) |
��2����Na2SO3��Һ����SO2����NaHSO3��
�ڳ����£���������pH=6ʱ����Һ�����ԣ�
��3��Pt��1���缫����SO2������Ũ������SO2��֪ʧ���ӣ���������ÿmolNO2�õ���4mol��
���
�⣺��1���ٴ�ͼ�пɿ���20minʱ�����Ũ�Ȳ��䣬��ʱ��Ӧ�ﵽƽ�⣬�ʴ�Ϊ��20��
��0��20minʱ��SO2Ũ�ȱ仯Ϊ1.0mol/L-0.4mol/L=0.6mol/L����Ӧ���ʱ�ʾΪV��SO2��=
=0.03mol/��L?min�����ʴ�Ϊ��0.03mol/��L?min����
��30minʱ��CO2Ũ�ȼ�С����������Ũ�Ȳ��䣬�ı�������Ǽ�СCO2��Ũ�ȣ�40minʱ��SO2��g��=0.4mol/L��S2��g��=0.35mol/L��CO2��g��=0.4mol/L����ƽ�ⳣ��K=
=
=0.35���ʴ�Ϊ����СCO2��Ũ�ȣ�0.35��
��2����Na2SO3��Һ����SO2�����ӷ���ʽΪSO32-+SO2+H2O=2HSO3-���ʴ�Ϊ��SO32-+SO2+H2O=2HSO3-��
�ڳ����£���������pH=6ʱ��
a�����ݵ���غ㣬c��Na+��+c��H+��=2c��SO32-��+c��HSO3-��+c��OH-��������c��Na+��+c��H+����c��SO32-��+c��HSO3-��+c��OH-������a����
b�����������غ㣬��������ȫΪNaHSO3ʱ��c��Na+��=c��SO32-��+c��HSO3-��+c��H2SO3������������Na2SO3����c��Na+����c��SO32-��+c��HSO3-��+c��H2SO3������b����
c��pH=6��c��OH-����c��H+������c����
d��HSO3-����̶ȴ���ˮ��̶ȣ�pH=6��ˮ�����c��OH-��=
=l��l0-8 mol/L����d��ȷ��
�ʴ�Ϊ��ad��
��3��SO2��Pt��1���缫ʧ��������SO42-���缫��ӦʽΪSO2+2H2O-2e-=SO42-+4H+��NO2��N�Ļ��ϼ�Ϊ+4�ۣ�ת��Ϊ0�۵�N2��ÿmolNO2�õ���4mol��������ת�Ƶ���6mol���������ϴ���NO2����Ϊ
=1.5mol��
�ʴ�Ϊ��SO2+2H2O-2e-=SO42-+4H+��1.5��
��0��20minʱ��SO2Ũ�ȱ仯Ϊ1.0mol/L-0.4mol/L=0.6mol/L����Ӧ���ʱ�ʾΪV��SO2��=
| 0.6mol/L |
| 20min |
��30minʱ��CO2Ũ�ȼ�С����������Ũ�Ȳ��䣬�ı�������Ǽ�СCO2��Ũ�ȣ�40minʱ��SO2��g��=0.4mol/L��S2��g��=0.35mol/L��CO2��g��=0.4mol/L����ƽ�ⳣ��K=
| c2(CO2)?c(S2) |
| c2(SO2) |
| 0��42��0.35 |
| 0��42 |
��2����Na2SO3��Һ����SO2�����ӷ���ʽΪSO32-+SO2+H2O=2HSO3-���ʴ�Ϊ��SO32-+SO2+H2O=2HSO3-��
�ڳ����£���������pH=6ʱ��
a�����ݵ���غ㣬c��Na+��+c��H+��=2c��SO32-��+c��HSO3-��+c��OH-��������c��Na+��+c��H+����c��SO32-��+c��HSO3-��+c��OH-������a����
b�����������غ㣬��������ȫΪNaHSO3ʱ��c��Na+��=c��SO32-��+c��HSO3-��+c��H2SO3������������Na2SO3����c��Na+����c��SO32-��+c��HSO3-��+c��H2SO3������b����
c��pH=6��c��OH-����c��H+������c����
d��HSO3-����̶ȴ���ˮ��̶ȣ�pH=6��ˮ�����c��OH-��=
| 1��10-14 |
| 1��10-6 |
�ʴ�Ϊ��ad��
��3��SO2��Pt��1���缫ʧ��������SO42-���缫��ӦʽΪSO2+2H2O-2e-=SO42-+4H+��NO2��N�Ļ��ϼ�Ϊ+4�ۣ�ת��Ϊ0�۵�N2��ÿmolNO2�õ���4mol��������ת�Ƶ���6mol���������ϴ���NO2����Ϊ
| 6 |
| 4 |
�ʴ�Ϊ��SO2+2H2O-2e-=SO42-+4H+��1.5��
���������⿼�黯ѧƽ����㣬��ѧƽ�ⳣ������ѧƽ��Ӱ�����ط��������ԭ���ķ���Ӧ�ã�����Ũ�ȴ�С�Ƚϣ��ۺ���ǿ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
A��B��C��D���ֽ�������AB�õ������Ӻ���ϡ�����У���A���������ų�����B�ܽ⣻�����ý������ں���A��C���ֽ������ӿ���������Һ��ʱ���ڻ��ý�����������C����D����B����������Һ�У���D������B�����������ֽ����Ļ����ǿ������˳���ǣ�������
| A��A��B��C��D |
| B��D��B��A��C |
| C��D��C��B��A |
| D��D��B��C��A |
�Ʊ�����������һ���ϳ�·��Ϊ������˵��������ǣ�������


| A���ҵĽṹ��ʽΪCH3COOCH2CH3 | |||
| B���Ҵ���CH3OCH3�������ѣ���Ϊͬ���칹�� | |||
| C����Ӧ�����ڼӳɷ�Ӧ | |||
D����Ӧ�ڵĻ�ѧ����ʽΪ2CH3CH2OH+O2
|
�����ձ���ѧ����һ��������ѧ�������������������л�����С��ü���������ϡ����������������ܵ������ʶ���2010��ŵ������ѧ��������˵����ȷ���ǣ�������
| A���л�����һ������̼��������Ԫ�� |
| B��һ�������£���������߷�Ӧ���ת���� |
| C�����л�����С���ϡ����Բ���ѭԭ���غ㶨�� |
| D�����л�����С��ü�����Ҫ�ƻ��ɵĻ�ѧ�� |
����ɢ������С��ͬ�ɽ���ɢϵ��Ϊ���塢��Һ����Һ����������СΪ��������
| A��0.01nm��1nm |
| B��0.1nm��10nm |
| C��1nm��100nm |
| D��10nm��1000nm |
�������ʵķ�������������IJ����غϵ��ǣ�������
| A��C2H2 |
| B��CO2 |
| C��NH3 |
| D��BF3 |
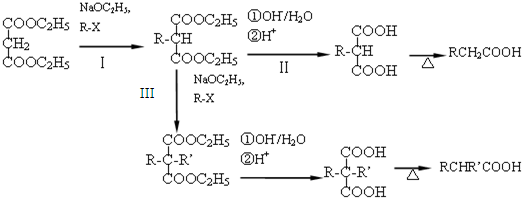
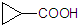 ������������Ľṹ��ʽΪ
������������Ľṹ��ʽΪ A��B��C��D����ǰ������Ԫ����ɵĵ��ʻ������֮����������ת����ϵ��
A��B��C��D����ǰ������Ԫ����ɵĵ��ʻ������֮����������ת����ϵ��