��Ŀ����
ij�о���ѧϰС��̽��FeSO4�Ļ�ѧ���ʺ���;���ش��������⣺
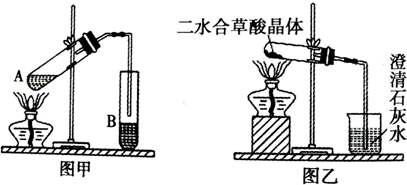
��һ��������ͼװ��̽��FeSO4���ȶ��Ժ���;�����̷��ɷ�ΪFeSO4?7H2O��
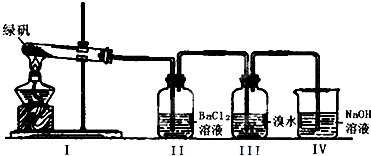
��1�������а�ɫ�������ɣ�˵��FeSO4����ֽ������� ��
A��Fe2O3����������B��FeO����������C��SO3 ����������D��SO2
��2��������ˮ�����������Ƿ���SO2�������ɣ��漰�Ļ�ѧ����ʽΪ ��ʵ���У��۲쵽��ˮ��ɫ���ݴ��Ʋ�FeSO4�ֽ����п��ܱ�������Ԫ���� ��
��3��NaOH������������SO2���壬��д����Ӧ��ѧ����ʽ�� ��
������̽��FeSO4��Fe2+��ԭ�ԣ�
��4��֤��FeSO4�н�ǿ�Ļ�ԭ�ԣ�����±��������������
����������������;̽��
��6��ȱ����ƶѪ�����ڷ������������������������Ƴ�ҩƬʱ���������һ����������£��������� ��
��һ��������ͼװ��̽��FeSO4���ȶ��Ժ���;�����̷��ɷ�ΪFeSO4?7H2O��

��1�������а�ɫ�������ɣ�˵��FeSO4����ֽ�������
A��Fe2O3����������B��FeO����������C��SO3 ����������D��SO2
��2��������ˮ�����������Ƿ���SO2�������ɣ��漰�Ļ�ѧ����ʽΪ
��3��NaOH������������SO2���壬��д����Ӧ��ѧ����ʽ��
������̽��FeSO4��Fe2+��ԭ�ԣ�
��4��֤��FeSO4�н�ǿ�Ļ�ԭ�ԣ�����±��������������
| ʵ�鲽�� | ʵ��Ԥ�������� |
| ����һ��ȡ������FeSO4�������Թ��У�����һ����ˮ�ܽ⣮ | \ |
| ������� |
��6��ȱ����ƶѪ�����ڷ������������������������Ƴ�ҩƬʱ���������һ����������£���������
���㣺����ʵ�鷽�������
ר�⣺ʵ����
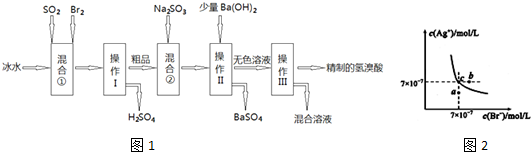
��������1�������������ȷֽ������ʹ�Ȼ�����Һ���ְ�ɫ��������ɫ����ֻ�������ᱵ����ѡ�������ʵ��������ش�
��2������������л�ԭ�ԣ�ͨ����ˮ���������嵥������Ϊ���
��3������������������Ʒ�Ӧ�����������ƺ�ˮ���ݴ���д����ʽ��
��4��FeSO4�н�ǿ�Ļ�ԭ�ԣ������ױ�����������Ϊ�������������������Ժ���������ӵĴ��ڼ��ɣ�
��6�����������������ױ������е������������ݴ˻ش�
��2������������л�ԭ�ԣ�ͨ����ˮ���������嵥������Ϊ���
��3������������������Ʒ�Ӧ�����������ƺ�ˮ���ݴ���д����ʽ��
��4��FeSO4�н�ǿ�Ļ�ԭ�ԣ������ױ�����������Ϊ�������������������Ժ���������ӵĴ��ڼ��ɣ�
��6�����������������ױ������е������������ݴ˻ش�
���
�⣺��1�������������ȷֽ������ʹ�Ȼ�����Һ���ְ�ɫ��������ɫ����ֻ�������ᱵ��˵���ֽ�����к���������������ѡ������ʺ��Ȼ�����Һ��϶������ʴ�Ϊ��C��
��2������������л�ԭ�ԣ�ͨ����ˮ���������嵥������Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽSO2+Br2+2H2O=H2SO4+2HBr���ʴ�Ϊ��SO2+Br2+2H2O=H2SO4+2HBr��
��3������������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ����ʽΪ2NaOH+SO2=Na2SO3+H2O���ʴ�Ϊ��2NaOH+SO2=Na2SO3+H2O��
��4��FeSO4�н�ǿ�Ļ�ԭ�ԣ������ױ�����������Ϊ�������������������Ժ���������ӵĴ��ڼ��ɣ������ǣ�ȡ������FeSO4�������Թ��У�����һ����ˮ�ܽ⣬�����м�����ˮ���ټ������軯����Һ�������Һ���ɫ������֤������������ǿ��ԭ�ԣ�
�ʴ�Ϊ��������ˮ���ٵ���KSCN��Һ����Һ��죬֤��������������ǿ�Ļ�ԭ�ԣ�
��6�����������������ױ������е�����������ȱ����ƶѪ�����ڷ������������������������Ƴ�ҩƬʱ���������һ����������£��������Ƿ�ֹ����������������
�ʴ�Ϊ����ֹ����������������
��2������������л�ԭ�ԣ�ͨ����ˮ���������嵥������Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽSO2+Br2+2H2O=H2SO4+2HBr���ʴ�Ϊ��SO2+Br2+2H2O=H2SO4+2HBr��
��3������������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ����ʽΪ2NaOH+SO2=Na2SO3+H2O���ʴ�Ϊ��2NaOH+SO2=Na2SO3+H2O��
��4��FeSO4�н�ǿ�Ļ�ԭ�ԣ������ױ�����������Ϊ�������������������Ժ���������ӵĴ��ڼ��ɣ������ǣ�ȡ������FeSO4�������Թ��У�����һ����ˮ�ܽ⣬�����м�����ˮ���ټ������軯����Һ�������Һ���ɫ������֤������������ǿ��ԭ�ԣ�
�ʴ�Ϊ��������ˮ���ٵ���KSCN��Һ����Һ��죬֤��������������ǿ�Ļ�ԭ�ԣ�
��6�����������������ױ������е�����������ȱ����ƶѪ�����ڷ������������������������Ƴ�ҩƬʱ���������һ����������£��������Ƿ�ֹ����������������
�ʴ�Ϊ����ֹ����������������
���������⿼��ѧ���������ӵ����ʡ������ӵļ����Լ�����֮��Ļ�ѧ����ʽ����д֪ʶ������ʵ�鷽�������̽���⣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ
���ڵ��ۺ���ά��[��C6H10O5��n]����������ȷ���ǣ�������
| A����Ϊͬ���칹�� |
| B��������Ȼ�л��߷��ӻ����� |
| C�����⣨I2���������ɫ |
| D���������ھ��ᷢ��ˮ�ⷴӦ |
����˵���У�������V��A��Ԫ�������������ǣ�������
| A�����γ�һ������ |
| B�����ϵ��µ��ʵ����������� |
| C�����ϵ����⻯��ķе������� |
| D�����ϵ����⻯����ȶ����� |