��Ŀ����
20����֪AΪ���ϩ�����߷��Ӳ���PET������֬��PMMA�ĺϳ�·�����£�
�����Ϣ���£�
��RCOOR��+R��18OH$��_{��}^{����}$RCO18OR��+R��OH��R��R�䡢R�����������
��RCOR��$��_{ii��H_{2}O/H+}^{i��HCN/OH-}$RCOHR��COOH��R��R�����������
��ش��������⣺
��1������˵����ȷ����BC������ĸ����
A��������F�ܷ���������Ӧ B��������E�뻯����D��Ϊͬϵ��
C��������G�ܷ������۷�Ӧ���ɾ��� D��1molPMMA������NaOH��Һ��Ӧ�������1molNaOH
��2����֪CH3OOCCOOCH3��ͬ���칹���ж��֣�д��ͬʱ��������������ͬ���칹��
 ��
�� ��
�� ��
�� ��
�����������ʵ���֮��1��2��NaHCO3��Һ������Ӧ�� �ں�����ױ��������к���һ����-CH3����
��1H-NMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ�ӣ�
��3��PET����Ľṹ��ʽΪ
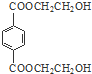 ��
����4��D+I��PMMA����Ļ�ѧ����ʽΪCH3OH+CH2=C��CH3��COOH$��_{��}^{Ũ����}$CH2=C��CH3��COOCH3+H2O��
��5�����A��B�ĺϳ�·�ߣ�������ͼ��ʾ�����Լ���ѡ����
���� ��PMMA�Ľṹ����֪PMMA����ΪCH2=C��CH3��COOCH3����D��I�ֱ�ΪCH2=C��CH3��COOH��CH3OH�е�һ�֣�AΪ���ϩ��ΪCH2=CH2����ϩ���巢���ӳɷ�Ӧ����CH2BrCH2Br��1��2-����������NaOHˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH��������ϢI��PET�������ʽ����֪PET����Ϊ ����DΪCH3OH��IΪCH2=C��CH3��COOH��PET���巢����ϢI�н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ
����DΪCH3OH��IΪCH2=C��CH3��COOH��PET���巢����ϢI�н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ ��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����GΪ
��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����GΪ ����FΪ
����FΪ ��EΪ
��EΪ ���ݴ˽��
���ݴ˽��
��� �⣺��PMMA�Ľṹ����֪PMMA����ΪCH2=C��CH3��COOCH3����D��I�ֱ�ΪCH2=C��CH3��COOH��CH3OH�е�һ�֣�AΪ���ϩ��ΪCH2=CH2����ϩ���巢���ӳɷ�Ӧ����CH2BrCH2Br��1��2-����������NaOHˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH��������ϢI��PET�������ʽ����֪PET����Ϊ ����DΪCH3OH��IΪCH2=C��CH3��COOH��PET���巢����ϢI�н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ
����DΪCH3OH��IΪCH2=C��CH3��COOH��PET���巢����ϢI�н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ ��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����GΪ
��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����GΪ ����FΪ
����FΪ ��EΪ
��EΪ ��
��
��1��A��������FΪ �������в���ȩ�������ܷ���������Ӧ����A����
�������в���ȩ�������ܷ���������Ӧ����A����
B��������EΪ �뻯����DΪCH3OH���ṹ��������Ȳ�CH2��Ϊͬϵ���B��ȷ��
�뻯����DΪCH3OH���ṹ��������Ȳ�CH2��Ϊͬϵ���B��ȷ��
C��������GΪ �������к����ǻ����Ȼ��ܷ������۷�Ӧ���ɾ�������C��ȷ��
�������к����ǻ����Ȼ��ܷ������۷�Ӧ���ɾ�������C��ȷ��
D���ṹ������֪1molPMMA�к���������ȷ����������NaOH��Һ��Ӧ�������NaOH��֪����D����
�ʴ�Ϊ��BC��
��2�����������ʵ���֮��1��2��NaHCO3��Һ������Ӧ��˵���������Ȼ���
�ں�����ױ��������к���һ����-CH3����
��1H-NMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ�ӣ����������б�����Ϊ����ȡ������λȡ����д����ͬ���칹��Ϊ�� ��
�� ��
�� ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
�� ��
�� ��
��
��3��PET����Ϊ�Զ��������Ҷ������ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4��DΪCH3OH��IΪCH2=C��CH3��COOH��PMMA�ĵ���Ϊ2-����ϩ�������D+I��PMMA����Ļ�ѧ����ʽΪ��CH3OH+CH2=C��CH3��COOH$��_{��}^{Ũ����}$CH2=C��CH3��COOCH3+H2O��
�ʴ�Ϊ��CH3OH+CH2=C��CH3��COOH$��_{��}^{Ũ����}$CH2=C��CH3��COOCH3+H2O��
��5������ϩ�ϳ��Ҷ������Ƚ���ϩ��������Ȼ�̼��Һ�����ӳɷ�Ӧ����������������Һ����ȡ����Ӧ���õ��Ҷ������ϳ�·��Ϊ��
CH2=CH2$\stackrel{Br/CCl_{4}}{��}$CH2Br-CH2Br$��_{��}^{NaOH/H_{2}O}$CH2OH-CH2OH��
��A��B�ĺϳ�·��ΪCH2=CH2$\stackrel{Br/CCl_{4}}{��}$CH2Br-CH2Br$��_{��}^{NaOH/H_{2}O}$CH2OH-CH2OH��
���� ���⿼���л�����ƶ���ϳɣ�������ø������Ϣ���л���Ľṹ�����ƶϣ���Ҫѧ���������չ����ŵ�������ת�����ϺõĿ���ѧ����ѧ������֪ʶǨ�����ã���Ŀ�Ѷ��еȣ�

| A�� | ʢ��NaOH��Һ���Լ�ƿ��ĥ�ڲ����� | |
| B�� | ����ᱣ��������ƿ�� | |
| C�� | �������Ʊ����ڹ��ƿ�� | |
| D�� | Ũ���ᱣ����ϸ���Լ�ƿ�� |

��֪��������I����Ҫ�ɷ���Fe2O3��MnO2��
�����������У������һ���⣬���ಽ�����ٵĻ��ϼ�δ�䣻
�۳���������������ˮ��
�ش��������⣺
��1�������Σ�FeWO4��MnWO4������Ԫ�صĻ��ϼ�Ϊ+6����д��MnWO4�����������·�����ֽⷴӦ����MnO2�Ļ�ѧ����ʽ2MnWO4+O2+4NaOH$\frac{\underline{\;����\;}}{\;}$2MnO2+2Na2WO4+2H2O��
��2�����������������������Һ�м������pH=10����Һ�е�����������ΪSiO32-��HAsO32-��HAsO42-��HPO42-�ȣ��������������У�����H2O2��Ŀ��������HAsO32-����HAsO42-�����������Ҫ�ɷ���MgSiO3��MgHAsO4��MgHPO4 ��
��3���������ܱ���������H2��ԭWO3�ɵõ������٣��䷴Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ�����
| �¶� | 25�桫550�桫600�桫700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
WO2��s��+2H2��g��?W��s��+2H2O��g����H=+66.0KJ•mol-1
WO2��s��?WO2��g����H=+203.9KJ•mol-1
����700��ʱ��WO2��g����H2��g����Ӧ���ɹ���W��s�����Ȼ�ѧ����ʽΪWO2��g��+2H2��g��?W��s��+2H2O��g��H=-137.9kJ•mol-1��
��4����֪�������ƺ�����ƣ�CaWO4���������Եĵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С����ͬ�¶����������ʵij����ܽ�ƽ��������ͼ2����T1ʱKsp��CaWO4��=1��10-10������������Һ�м���ʯ����õ���������ƣ���T2ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ1��103��
��5�����õ�ⷨ���Դ�̼���٣�WC�������л����٣����ʱ����̼�����������������������������Ϊ���Һ�������������Ტ�ų�CO2����������ӦʽΪWC+6H2O-10e-=H2WO4+CO2��+10H+��
 ��ͼ�Dz��ֶ�����Ԫ��ԭ�ӣ�����ĸ��ʾ��������������ԭ�������Ĺ�ϵͼ������˵����ȷ���ǣ�������
��ͼ�Dz��ֶ�����Ԫ��ԭ�ӣ�����ĸ��ʾ��������������ԭ�������Ĺ�ϵͼ������˵����ȷ���ǣ�������| A�� | X��W�γɵĻ�������ֻ�й��ۼ� | B�� | X��Z�γɵĻ�������ֻ�����Ӽ� | ||
| C�� | Ԫ�صķǽ����ԣ�X��R��W | D�� | �����ӵİ뾶��W��R��X |
| A�� | d�ĺ�������ǿ�� | |
| B�� | ���Ӱ뾶��d��c��b | |
| C�� | b��c��d�γɵĻ�������ֻ�����Ӽ� | |
| D�� | a��c�γɵ����ӻ�������л�ԭ�ԣ�����ˮ��Ӧ |
| A�� | ��ˮ����ʱ�õ��ȿ�����Br2���� | B�� | ��ҵ��HClʱ������������ȼ�� | ||
| C�� | ���Ṥҵ��ʹ�õ��Ƚ����� | D�� | ʯ��ͨ������õ��ѻ����� |
��֪������������������������ʽ����ʱ��Һ��pH���±���

| ������ | Fe��OH��3 | Fe��OH��2 | Al��OH��3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
��1����ӦI��Һ�д��ڵĽ�����������Fe2+��Al3+��
��2������NaHCO3��Ŀ���ǵ���pH��ʹ��Һ�е�Al3+���Fe3+������Fe2+����A13+�����������ù��������С����衱�������Ǽӿ췴Ӧ���ʣ�
��3����ӦII�����ӷ���ʽΪ2H++Fe2++NO2-=Fe3++NO��+H2O����ʵ�������У���ӦII��ͬʱͨ��O2�Լ���NaNO2��������O2��NaNO2�ڷ�Ӧ�о���Ϊ�������������뷴Ӧ��O2��11.2L����״���������൱�ڽ�ԼNaNO2���ʵ���Ϊ2mol��
��4����ʽ����������ˮ�������[Fe��OH��]2+���ӣ��ɲ���ˮ������${[{F{e_2}{{��{OH}��}_4}}]^{2+}}$�ۺ����ӣ���ˮ�ⷴӦ�����ӷ���ʽΪ2Fe��OH��2++2H2O?Fe2��OH��42++2H+��
| A�� | 20mx | B�� | 20x/m | C�� | 25mx | D�� | 50m/x |
��CaCl2��Һ
��Na2SiO3��Һ
��Ca��ClO��2��Һ
�ܱ���Na2CO3��Һ
��NaAlO2 �����г����������ǣ�������
| A�� | �ڢܢ� | B�� | �٢ڢۢ� | C�� | �ڢۢܢ� | D�� | �ڢۢ� |