��Ŀ����
13����һƿ�������Һ�����п��ܺ���H+��NH4+��Mg2+��Ba2+��I-��CO32-��SO42-��ȡ����Һ��������ʵ�飺��1��ȡpH��ֽ���飬��Һ�����ԣ�
��2��ȡ��������Һ����������CCl4������������ˮ������CCl4���Ϻ�ɫ��
��3����ȡ��������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ���Ӧ�����о�����������
��4��ȡ������������3���м�����Һ��Na2CO3��Һ���а�ɫ�������ɣ�
��������ʵ����ʵ����ȷ��������Һ�У�
�϶����ڵ�������H+��I-��Ba2+���϶������ڵ�������CO32-��Mg2+��SO42-��������ȷ���Ƿ���ڵ�������NH4+��
���� ��1����pH��ֽ���飬������Һ����ǿ���ԣ�һ������H+��CO32-�ܹ��������ӷ�Ӧ������Һ�в�����ڣ�
��2�����Ȼ�̼��Һ���Ϻ�ɫ��˵��������ˮ���еⵥ�����ɣ�ԭ��Һ��һ������I-��
��3����������������Һ�Ĺ�����û�г������ɣ�˵��һ��������Mg2+��
��4��Ba2+����̼���Ʒ�Ӧ�����������жϴ��ڵ����ӣ�һ������Ba2+���������ӹ����жϲ��ܴ��ڵ����ӣ�
��� �⣺��1����pH��ֽ���飬������Һ����ǿ���ԣ�һ������H+��CO32-�ܹ��������ӷ�Ӧ������Һ�в�����ڣ�
��2�����Ȼ�̼��Һ���Ϻ�ɫ��˵��������ˮ���еⵥ�����ɣ�ԭ��Һ��һ������I-��
��3����������������Һ�Ĺ�����û�г������ɣ�˵��һ��������Mg2+��
��4��Ba2+����̼���Ʒ�Ӧ�����������жϴ��ڵ����ӣ�һ������Ba2+��һ��������CO32-��SO42-��NH4+���ж��Ƿ���ڣ�
�ʴ�Ϊ��H+��I-��Ba2+��CO32-��Mg2+��SO42-��NH4+��
���� ������Ҫ�����˳������ӵļ��鷽������Ŀ�Ѷ��еȣ�ע�����ճ������ӵĻ�ѧ���ʼ����鷽�����ܹ��������ӹ��桢���ӷ�Ӧ�����ж����ӹ����������ȷ��������ʱ�������ų��������ӣ�ȷ�����鷽���������ԣ�

 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�| A�� | Na | B�� | Fe | C�� | Cu | D�� | Al |
| A�� | A��B�ֱ�Ϊ0.4mol•L-1��0.2mol•L-1 | B�� | AΪ0.25mol•L-1 | ||
| C�� | A��C��Ϊ0.15mol•L-1 | D�� | AΪ0.24mol•L-1��CΪ0.14mol•L-1 |
��1����һ�ݼ����������ᣬ���κ����������
��2���ڶ��ݼ�������NaOH��Һ�������衢���ˡ�ϴ�ӡ����ա����õ�����ɫ���壻
��3�������ݵμ�0.1mol��L-1����KMnO4��Һ��KMnO4��Һ����ɫ��ʧ
��4�����ýྻ�IJ�˿պȡ�û����Һ����dzɫ���������գ����ֻ�����ֻ�ɫ��
��������ʵ�飬����˵����ȷ���ǣ�������
| A�� | ԭ�����Һ��ֻ����Na+��Fe3+��SO42-�������ܴ���K+��CO32- | |
| B�� | ��ʵ�飨1�����ƶ�ԭ�����Һ���Ƿ���SO42- | |
| C�� | ��ʵ�飨2�����ƶ�ԭ�����Һ���Ƿ���Fe3+ | |
| D�� | ��ʵ�飨3�����ƶ�ԭ�����Һ�д���Fe2+ |
| A�� | HCl | B�� | NaOH | C�� | Na2SO4 | D�� | NaCl |
 ���붬��������ʼ��ů�����������������أ�����PM2.5��PM10������������������������������Ⱦ���У���Ϊ�������ǻ�����β���еĵ��������ȼú������������
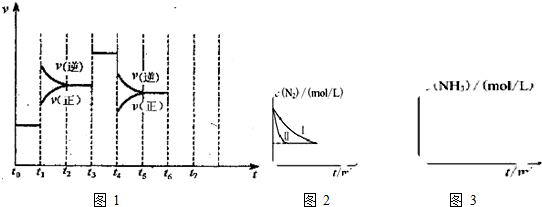
���붬��������ʼ��ů�����������������أ�����PM2.5��PM10������������������������������Ⱦ���У���Ϊ�������ǻ�����β���еĵ��������ȼú����������������֪��ӦN2O4��g��?2NO2��g����H�����¶����ߣ�����������ɫ�����һ����N2O4��������������һ��ʱ����о�ѹ����������������л���������������ʣ�������ɫԽdz������Խ����ʱ��仯���������˵����˵�����ʲ��ٷ����ı����ad��
a��������ɫ���ٸı� b����H���ٸı�
c��v����N2O4��=2v����NO2�� d��N2O4��ת���ʲ��ٸı�
����NH3����ԭNOx�������������������Ⱦ������ͼ������NH3����ԭ����������һ��������ͨ�����ֲ�ͬ�����������ݳ������е������ﺬ�����Ӷ�ȷ�������ѵ��ʣ�ע���ѵ��ʼ���������ת���ʣ�����Ӧԭ��Ϊ��
NO��g��+NO2��g��+2NH3��g��?2N2��g��+3H2O��g����
��1���÷�Ӧ�ġ�S��0�����������=����������
��2������˵����ȷ����C��
A���ڢ��ִ����ȵڢ��ִ����ѵ��ʸ�
B����ͬ�����£��ı�ѹǿ���ѵ���û��Ӱ��
C�������١��ڷֱ��ʺ���250���450�������ѵ�
����CH4����ԭNOxҲ�������������������Ⱦ��
��3����֪��CH4��g���ı�ȼ����Ϊ-890kJ/mol������1mol H2O��l����Ҫ����44kJ������
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1114kJ/mol
2NO��g��+O2��g���T2NO2��g����H=-114kJ/mol
д��CH4����ԭNO2��g������N2��H2O��g�����Ȼ�ѧ����ʽ��CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-844kJ/mol��
��4�����¶�ΪT1���T2��ʱ���ֱ�0.5mol CH4��1.2mol NO2�������Ϊ1L���ܱ������У����NO2�����ʵ�����ʱ��仯�������±���
ʱ��/min �¶�/�� | 0 | 10 | 20 | 40 | 50 |
| T1 | 1.2 | 0.9 | 0.7 | 0.4 | 0.4 |
| T2 | 1.2 | 0.8 | 0.56 | �� | 0.5 |
��T1��T2��������������¿�ͬ�����ж������������¶ȣ�NO2�����ʵ�������ƽ�������ƶ�������ӦΪ���ȷ�Ӧ��
��T1��ʱ����ӦCH4��g��+2NO2��g��?N2��g��+CO2��g��+2H2O��g��ƽ�ⳣ��K=6.4��
���¶�ΪT2��ʱ����ƽ������������м���0.5mol CH4��1.2mol NO2������ƽ��ʱCH4��ת���ʽ���С�����������С�����䡱����