��Ŀ����
4��NH3��NO2�dz����ĵ��Ļ�����о����ǵ��ۺ���������Ҫ���壮��1��NO2�����Ļ��������в����⻯ѧ�������γ����꣨�����֣���
��2���ȵ糧ͨ����NH3����ȼú������NO2���÷�Ӧ��ѧ����ʽ8NH3+6NO2$\frac{\underline{\;һ������\;}}{\;}$7N2+12H2O��
��3���������Ȼ��������ϵ����÷�Ӧ����;�Dz������̣����鰱�����Ȼ����Ƿ�й©��
��4�������������������������ϣ�������һ�������������һ�ֻ�����A��A������ԭ�Ӿ�����8�����ȶ��ṹ����д��A�ĵ���ʽ
 ��
��
���� ��1�����ݺ���������Ի�����Ӱ�����ش𣬵�����������Ⱦ�����壬�����γɹ⻯ѧ������
��2���������л�ԭ�ԣ�NO2����ǿ�����ԣ��������������������ɵ�����ˮ��
��3���������Ȼ��������Ϸ�Ӧ���ɰ�ɫ�����Ȼ�泥���Ӧ����ð���̣����ݴ�������Լ��鰱�����Ȼ����Ƿ�й©��
��4�������������������������ϣ�������һ�������һ�ֻ�����A��A������ԭ�Ӿ�����8�����ȶ��ṹ�����ɵ�����HClO��AΪNCl3��
��� �⣺��1�����������������������γ����������꣬�������γɹ⻯ѧ������
�ʴ�Ϊ�������⻯ѧ�������γ����ꣻ
��2���������л�ԭ�ԣ�NO2����ǿ�����ԣ��������������������ɵ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��8NH3+6NO2$\frac{\underline{\;һ������\;}}{\;}$7N2+12H2O��
�ʴ�Ϊ��8NH3+6NO2$\frac{\underline{\;һ������\;}}{\;}$7N2+12H2O��
��3���������Ȼ��������Ϸ�Ӧ���ɰ�ɫ�����Ȼ�泥�NH3+HCl=NH4Cl����Ӧ����������̣����ݴ�������Լ��鰱�����Ȼ����Ƿ�й©��
�ʴ�Ϊ���������̣����鰱�����Ȼ����Ƿ�й©��
��4�������������������������ϣ�������һ�������һ�ֻ�����A��A������ԭ�Ӿ�����8�����ȶ��ṹ�����ɵ�����HClO��AΪNCl3������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�鰱�����������ʣ����ؿ���ѧ�������ƶ�������֪�������ļ��鷽���������������������ǽ���ؼ�����Ŀ�ѶȲ���

 | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
2 | N | O | F | |||||
3 | Na | Mg | Al | S | Cl | Ar | ||
4 | K |
��2��Sԭ�ӽṹʾ��ͼ
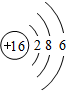 ��
����3��N��F��Cl�У�ԭ�Ӱ뾶������Cl��
��4������������Ӧˮ����������ǿ���Ǹ����ᣨ�����ƣ���
��5��д��ʵ���������������ӷ���ʽMnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
 ij��ɫϡ��ҺX�У����ܺ���������������е�ij���֣�
ij��ɫϡ��ҺX�У����ܺ���������������е�ij���֣�| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Fe3+��Mg2+��NH4+��Na+ |
| A�� | ��Y�����ᣬ��X��һ������CO32-��SiO32-��AlO2-��Na+ | |
| B�� | ��Y��NaOH��Һ����X��һ������Al3+��Fe3+��NH4+��Cl- | |
| C�� | ��Y��NaOH��Һ����ab�η�����Ӧ�����ӷ���ʽΪ��NH4++OH-�TNH3��+H2O | |
| D�� | ��Y��NaOH��Һ����X�е�Al3+��Mg2+��NH4+ ���ʵ���֮��Ϊ1��1��2 |
 ��1����֪��3H2��g��+3CO��g���TCH3OCH3��g��+CO2��g������H=-247kJ/mol��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��AE��
��1����֪��3H2��g��+3CO��g���TCH3OCH3��g��+CO2��g������H=-247kJ/mol��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��AE��a�����¸�ѹ b��������� c�����������뺤��
d������CO��Ũ�� e�������������
��2����֪��Ӧ2CH3OH��g���TCH3OCH3��g��+H2O��g������ij�¶��£���1L�ܱ������м���CH3OH����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.01 | 0.2 | 0.2 |
��������CH3OH��10min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v��CH3OH��=0.04 mol•L-1•min-1����ƽ��������������ټ���0.01mol CH3OH��0.2mol CH3OCH3����ʱ�����淴Ӧ���ʵĴ�С��v���� v�� �����������������=������
��3���״�������Ϊ��Ҫ���л�ȼ�ϣ�ͨ������CO��H2�ϳɼ״����䷴Ӧ�Ļ�ѧ����ʽΪCO��g��+2H2��g���TCH3OH��g��������һ�ݻ��ɱ���ܱ������У�����10mol CO��20mol H2�����ںϳɼ״���CO��ƽ��ת���ʣ��������¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ��ʾ��
�������ϳɼ״��ķ�ӦΪ���ȷ�Ӧ������ȡ������ȡ�����
��A��B��C�����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵΪKA=KB��KC��
�������ﵽƽ��״̬Aʱ���ɵļ״����ڹ��ɼ״�һ����ȼ�ϵ�أ��������ҺΪKOHŨ��Һ����õ�ع���ʱ�����ĵ缫��ӦʽΪO2+2H2O+4e-=4OH-��
| A�� | ��ϩ | B�� | �Զ��ױ� | C�� |  | D�� |  |
| A�� | ij������ɫ��Ӧ�ʻ�ɫ�����ۣ�������һ�������� | |
| B�� | ij��Һ�еμ�KSCN��Һ����Һ����죬�ٵμ���ˮ����죬���ۣ�ԭ��Һһ����Fe2+ | |
| C�� | ��ɫ��Һ�м���BaCl2��Һ���а�ɫ�����������ټ�ϡ���ᣬ��������ʧ�����ۣ�ԭ��Һһ������SO42- | |
| D�� | ��ɫ��Һ�м���ϡ���ᣬ������ɫ��ζ���壬��������ʹ����ʯ��ˮ����ǣ����ۣ�ԭ��Һһ������CO32- |
| A�� | ƽ��ʱSO2��ת���ʣ�A��B | |
| B�� | ���淴Ӧ�ӿ�ʼ��ƽ��ų���������A��B | |
| C�� | ƽ��ʱ�������������ʵ�����A��B | |
| D�� | ƽ��ʱ�Ļ�ѧ��Ӧ���ʣ�A��B |
| A�� |  ������̼ԭ�ӿ�����ͬһƽ���� ������̼ԭ�ӿ�����ͬһƽ���� | |
| B�� |  ������Ϊ2-��-1-���� ������Ϊ2-��-1-���� | |
| C�� | ��ϩ��������ϩ�ͱ������о�����̼̼˫�� | |
| D�� | C4H8����ϩ����ͬ���칹�干��4�֣���˳���칹�� |