��Ŀ����
������ѡ����
����˵����ȷ���� ��
A������B3N3H6��Ϊ�ȵ����壬�ҷ�����ԭ�ӹ�ƽ��
B����ϩ�Ͷ�����̼��̼ԭ�Ӿ�����sp2�ӻ�
C��NC13�ǷǼ��Է��ӣ�H2O2�Ǽ��Է���
D��P��S���ڵ�������Ԫ�أ�S�ĵ縺��ǿ��P
�����������ߵ�ֱ��Ӱ�������������������ܹ���Ⱦ�����ijɷ��ж��֣���������SO2��CO��NO2��O3�Ϳ����������ȣ���ش��������⣺
��1��S��N��O�ĵ�һ�������ɴ�С��˳��Ϊ ��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������ ���壮
��3����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е���-19.5�棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е��� 64.65�棩����ȩ������Cԭ�Ӳ�ȡ �ӻ������ʽ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���� ��
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu��CO��Cl?H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ ��
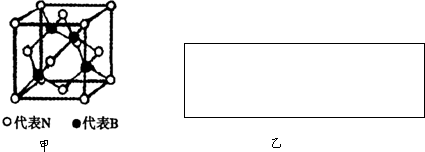
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl-�������������Cu+�� ��������CuCl�����е�Cu+���Ӻ�Cl-����ȫ������Cԭ�ӣ����Ϊ���ʯ�ľ�������һ�����ʯ�����к��� Cԭ�ӣ�

��Cu��CO��Cl?H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ�������6������λ��������ͼ���ü�ͷ��ʾ����
����˵����ȷ����
A������B3N3H6��Ϊ�ȵ����壬�ҷ�����ԭ�ӹ�ƽ��
B����ϩ�Ͷ�����̼��̼ԭ�Ӿ�����sp2�ӻ�
C��NC13�ǷǼ��Է��ӣ�H2O2�Ǽ��Է���
D��P��S���ڵ�������Ԫ�أ�S�ĵ縺��ǿ��P
�����������ߵ�ֱ��Ӱ�������������������ܹ���Ⱦ�����ijɷ��ж��֣���������SO2��CO��NO2��O3�Ϳ����������ȣ���ش��������⣺
��1��S��N��O�ĵ�һ�������ɴ�С��˳��Ϊ
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������
��3����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е���-19.5�棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е��� 64.65�棩����ȩ������Cԭ�Ӳ�ȡ
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu��CO��Cl?H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl-�������������Cu+��

��Cu��CO��Cl?H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ�������6������λ��������ͼ���ü�ͷ��ʾ����
���㣺�����ļ���,ԭ�Ӻ�������Ų�,Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ��,�����ijɼ����
ר�⣺ԭ�������ṹר��,��ѧ���뾧��ṹ
����������A���۵���������ȣ�ԭ����Ҳ��Ȼ�Ϊ�ȵ����壬�ȵ�����ṹ���ƣ�
B����������ԭ���γɵĦļ��µ��Ӷ����ж���ϩ�Ͷ�����̼��̼ԭ�ӵ��ӻ����ͣ�
C���жϷ��ӿռ�ṹ���ӽṹ�ԳƵĽǶ��ж�������������Ƿ��ص���
D��ͬ����������ҵ縺����ǿ��
����1��ͬһ���ڣ�Ԫ�صĵ�һ����������ԭ�����������������������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�ͬһ���壬Ԫ�صĵ�һ����������ԭ���������������С��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ�����ڷ��Ӿ��壻
��3����ȩ�ĽṹΪ ������Cԭ���γɵĦļ��µ��Ӷ����ж�Cԭ�ӵ��ӻ���ʽ����������������ʵķе���죻
������Cԭ���γɵĦļ��µ��Ӷ����ж�Cԭ�ӵ��ӻ���ʽ����������������ʵķе���죻
��4����ͭԭ�Ӻ��������Ϊ29�����ݹ���ԭ����д��̬�����Ų�ʽ��
�ڴ�CuCl�ľ��������жϣ���Cu+����������ڵ�Cl-��4������Ϊ1��1�ͽṹ������Cl-����������ڵ�Cu+��4�������ݾ�̯������ṹʽ������Cԭ����Ŀ��
��Cu��CO��H2O������һ��Clԭ���γ���λ����
B����������ԭ���γɵĦļ��µ��Ӷ����ж���ϩ�Ͷ�����̼��̼ԭ�ӵ��ӻ����ͣ�
C���жϷ��ӿռ�ṹ���ӽṹ�ԳƵĽǶ��ж�������������Ƿ��ص���
D��ͬ����������ҵ縺����ǿ��
����1��ͬһ���ڣ�Ԫ�صĵ�һ����������ԭ�����������������������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�ͬһ���壬Ԫ�صĵ�һ����������ԭ���������������С��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ�����ڷ��Ӿ��壻
��3����ȩ�ĽṹΪ
 ������Cԭ���γɵĦļ��µ��Ӷ����ж�Cԭ�ӵ��ӻ���ʽ����������������ʵķе���죻
������Cԭ���γɵĦļ��µ��Ӷ����ж�Cԭ�ӵ��ӻ���ʽ����������������ʵķе���죻��4����ͭԭ�Ӻ��������Ϊ29�����ݹ���ԭ����д��̬�����Ų�ʽ��
�ڴ�CuCl�ľ��������жϣ���Cu+����������ڵ�Cl-��4������Ϊ1��1�ͽṹ������Cl-����������ڵ�Cu+��4�������ݾ�̯������ṹʽ������Cԭ����Ŀ��
��Cu��CO��H2O������һ��Clԭ���γ���λ����
���
�⣺����A������B3N3H6�۵���������ȣ�ԭ����Ҳ��Ȼ�Ϊ�ȵ����壬��ΧŹƽ��ṹ��������ԭ�ӹ�ƽ�棬��A��ȷ��
B����ϩ��������̼��̼ԭ�ӷֱ��γ�3��2���ļ�����û�йµ��Ӷԣ���ֱ����sp2��sp�ӻ�����B����
C��NC13������Nԭ�ӳ�3���ļ�����1�Թµ��Ӷԣ�Ϊ�����ͣ����ǶԳƽṹ�����ڼ��Է��ӣ�H2O2��Oԭ�ӳ�2���ļ�����2�Թµ��Ӷԣ�O��ԭ�����ӵ���ԭ�ӡ�Hԭ���γ�V�ͣ���������Ϊչ����ҳ�ͽṹ�����ǶԳƽṹ�����ڼ��Է��ӣ���C����
D��P��S���ڵ�������Ԫ�أ�ͬ����������ҵ縺����ǿ����S�ĵ縺��ǿ��P����D��ȷ��
��ѡ��AD��
����1��ͬһ���ڣ�Ԫ�صĵ�һ����������ԭ�����������������������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�ͬһ���壬Ԫ�صĵ�һ����������ԭ���������������С�����Ե�һ�����ܴ�С˳���ǣ�N��O��S���ʴ�Ϊ��N��O��S��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ�����ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��3���ɼ�ȩ�Ľṹ ��֪��̼ԭ���ϵŶԵ�����Ϊ0���Ҽ���Ϊ3����̼ԭ�ӵ��ӻ�����Ϊsp2����״�����֮�������γɷ��Ӽ��������е�ߣ�����ȩ�в����������
��֪��̼ԭ���ϵŶԵ�����Ϊ0���Ҽ���Ϊ3����̼ԭ�ӵ��ӻ�����Ϊsp2����״�����֮�������γɷ��Ӽ��������е�ߣ�����ȩ�в����������
�ʴ�Ϊ��sp2���״����Ӽ�������������ȩû�У�
��4����ͭԭ�Ӻ��������Ϊ29����̬�����Ų�ʽʽΪ1s22s22p63s23p63d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��
�ڴ�CuCl�ľ��������жϣ���Cu+����������ڵ�Cl-��4������Ϊ1��1�ͽṹ������Cl-����������ڵ�Cu+��4�������ʯ��������4��Cԭ��Ϊ�����ڲ���8��Cԭ��λ�ڶ��㡢6��Cԭ����Χ���ģ��ʽ��ʯ������Cԭ����Ŀ=4+8��
+6��
=8���ʴ�Ϊ��4��8��
��Cu��CO��H2O������һ��Clԭ���γ���λ������ͼ��ʾ ��
��
�ʴ�Ϊ�� ��
��
B����ϩ��������̼��̼ԭ�ӷֱ��γ�3��2���ļ�����û�йµ��Ӷԣ���ֱ����sp2��sp�ӻ�����B����
C��NC13������Nԭ�ӳ�3���ļ�����1�Թµ��Ӷԣ�Ϊ�����ͣ����ǶԳƽṹ�����ڼ��Է��ӣ�H2O2��Oԭ�ӳ�2���ļ�����2�Թµ��Ӷԣ�O��ԭ�����ӵ���ԭ�ӡ�Hԭ���γ�V�ͣ���������Ϊչ����ҳ�ͽṹ�����ǶԳƽṹ�����ڼ��Է��ӣ���C����
D��P��S���ڵ�������Ԫ�أ�ͬ����������ҵ縺����ǿ����S�ĵ縺��ǿ��P����D��ȷ��
��ѡ��AD��
����1��ͬһ���ڣ�Ԫ�صĵ�һ����������ԭ�����������������������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�ͬһ���壬Ԫ�صĵ�һ����������ԭ���������������С�����Ե�һ�����ܴ�С˳���ǣ�N��O��S���ʴ�Ϊ��N��O��S��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ�����ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��3���ɼ�ȩ�Ľṹ
 ��֪��̼ԭ���ϵŶԵ�����Ϊ0���Ҽ���Ϊ3����̼ԭ�ӵ��ӻ�����Ϊsp2����״�����֮�������γɷ��Ӽ��������е�ߣ�����ȩ�в����������
��֪��̼ԭ���ϵŶԵ�����Ϊ0���Ҽ���Ϊ3����̼ԭ�ӵ��ӻ�����Ϊsp2����״�����֮�������γɷ��Ӽ��������е�ߣ�����ȩ�в�����������ʴ�Ϊ��sp2���״����Ӽ�������������ȩû�У�
��4����ͭԭ�Ӻ��������Ϊ29����̬�����Ų�ʽʽΪ1s22s22p63s23p63d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��
�ڴ�CuCl�ľ��������жϣ���Cu+����������ڵ�Cl-��4������Ϊ1��1�ͽṹ������Cl-����������ڵ�Cu+��4�������ʯ��������4��Cԭ��Ϊ�����ڲ���8��Cԭ��λ�ڶ��㡢6��Cԭ����Χ���ģ��ʽ��ʯ������Cԭ����Ŀ=4+8��
| 1 |
| 8 |
| 1 |
| 2 |
��Cu��CO��H2O������һ��Clԭ���γ���λ������ͼ��ʾ
 ��
���ʴ�Ϊ��
 ��
��
���������⿼���Ϊ�ۺϣ��漰�����Ų�ʽ�������ܡ����ӵ����幹�͡��ӻ�������͡��������λ�������Լ�����ṹ������֪ʶ����Ŀ�Ѷ��еȣ�ע�⣨4���н��CuCl�Ļ�ѧʽ�жϾ��������������Ŀ��

��ϰ��ϵ�д�
�����Ŀ
�����ӵ�����ԼΪ6.02��1023mol-1������������ȷ���ǣ�������
| A��25��ʱ��1L pH=13��NaOH��Һ��Լ����6.02��1023������������ |
| B��4.6g Na�ڿ�������ȫ��Ӧ����Na2O��Na2O2��ת��Լ0.2��6.02��1023������ |
| C��1mol�ǻ���-OH�������ĵ�����ԼΪ10��6.02��1023 |
| D����״���£����һ������ͭ�����ᷴӦ������22.4L�Ļ�����壬��ԭ������ķ�����Ӧ����6.02��1023 |
��ͬ��ͬѹ�£��ѵ�����Ŀ����Ͷ�����̼��ϣ��ڸ����¸������Ľ�̿��ַ�Ӧ���������Ͷ�����̼��ת��Ϊһ����̼����Ӧ��������һ����̼���������Լ�ǣ�������
| A��0.75 | B��0.70 |
| C��0.64 | D��0.60 |
ij��ҵ��ˮ�ܿ��ٸ�ʴ����ʯ���ù�ҵ��ˮ�У��ɳ���������������ǣ�������
| A��Na+��Mg2+��ClO-��NO3- |
| B��Al3+��NH4+��Br-��Cl- |
| C��K+��MnO4-��Fe2+��SO42- |
| D��Na+��K+��SiO32-��Cl- |

 MnO2�Ǽ��̵�ز���������ͨ����������֮һ����MnO2�м���CoTiO3�����壬��������������ʣ��Ż����̵�ص����ܣ�
MnO2�Ǽ��̵�ز���������ͨ����������֮һ����MnO2�м���CoTiO3�����壬��������������ʣ��Ż����̵�ص����ܣ� ��������PTCԪ������Ҫ�ɷ�Ϊ���ѿ��侧��ṹ��ͼ��ʾ���ýṹ�Ǿ��д����Ե���С�ظ���λ���þ��徭X���߷����������ظ���λΪ�����壬�߳�Ϊ403.1pm������λ��ΪTi4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��
��������PTCԪ������Ҫ�ɷ�Ϊ���ѿ��侧��ṹ��ͼ��ʾ���ýṹ�Ǿ��д����Ե���С�ظ���λ���þ��徭X���߷����������ظ���λΪ�����壬�߳�Ϊ403.1pm������λ��ΪTi4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��
