��Ŀ����
9������Na2O2��ˮ��Ӧ�ܷų����������ʣ���ͨ��ѡ��ͼ1װ��A��B���ⶨ�Ѳ��ֱ��ʵ�Na2O2��Ʒ��Na2O2������������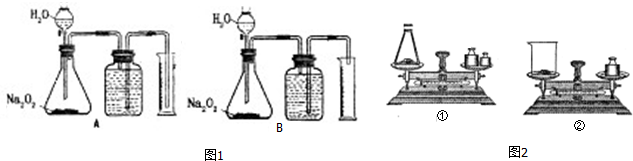
��1��ʵ�����ṩ����500mL��Ͳ����ʵ����ȡ��Na2O2��Ʒ����������ʵ���B��
A��0.1��0.2g B��2.5��3.0g C��5.0��6.0g D��10��15g
��2����ͼ������ƽ��ȡ��Ʒ��Ӧѡ��ͼ2�еĢ٣���ٻ�ڣ�����ѡ����һװ�õ�ԭ���ǹ��������������տ����е�H2O��CO2�����˱�¶�ڿ����г��������������������տ����еĶ�����̼��ˮ���������˱�¶�ڿ����г�����
��3����Na2O2��ˮ��Ӧ����ȶ�Ӱ��ⶨ��ȷ�ԣ����Է�Ӧ��������ʹ��ƿ�е������¶Ȼָ������£�Ӧѡ��װ��A��B�е�A����A��B�������ѡ������һ�ֲ��ʵ���װ�ã���õ�Na2O2������������ƫ����ƫ���ƫС������Ͳ��ˮ�������������ʱ�ų������������
��4������ʵ���У�����Ħ�����Ϊa L•mol-1����Ͳ���ռ�����ˮ�����ΪV mL����Ʒ������Ϊm g������Ʒ��Na2O2����������Ϊ$\frac{15.6V}{am}$%��
���� ��1��500mL��Ͳ��ȡ���ǹ���������ˮ��Ӧ�������������������500mL�������������������Ƶ����������
��2�����������������տ����е�H2O��CO2�����˱�¶�ڿ����г������ձ��й�������������Ӵ������
��3����Na2O2��ˮ��Ӧ����ȶ�ʹ�����������������¹������Ƶ���������ƫ��Ӧ��ȴ���壬ѡ�ó����ܣ�
��4����Ͳ���ռ�����Һ�������ΪO2������������������������������Ƶ�����
��� �⣺��1��500mL��Ͳ��ȡ���ǹ���������ˮ��Ӧ������������������ɵ�500mL��������ԼΪ0.022mol������2Na2O2+2H2O=4NaOH+O2������Ҫ������������ԼΪ0.044mol��78g/mol=3.4g��Na2O2������ӦС��3.4g���������������Ͳ��Һ�����磬������ٲ���������̫�٣�������������Ͳ�����������̫��ѡ��Na2O2��Ʒ�ĺ�������Ϊ2.5g��3.0g��
�ʴ�Ϊ��B��
��2�����������������տ����е�H2O��CO2�����˱�¶�ڿ����г������ձ��й�������������Ӵ����������ѡ������ƿ��ȡ��
�ʴ�Ϊ���٣����������������տ����е�H2O��CO2�����˱�¶�ڿ����г�����
��3����Na2O2��ˮ��Ӧ����ȶ�ʹ�����������������¹������Ƶ���������ƫ��Ӧ��ȴ���壬ѡ�ó����ܣ���ֹ��Ͳ�Ķ�����������ʱ�ų��������������
�ʴ�Ϊ��A��ƫ��
��4����Ͳ���ռ�����Һ�������ΪO2������������ʵ���n��O2��=$\frac{{10}^{-3}V}{a}$mol�����ݷ�Ӧ��ϵʽ��2Na2O2��O2�����ɵ�n��Na2O2��=2��$\frac{{10}^{-3}V}{a}$mol��Na2O2����������=$\frac{2��\frac{{10}^{-3}V}{a}mol��78g/mol}{mg}$��100%=$\frac{15.6V}{am}$%��
�ʴ�Ϊ��$\frac{15.6V}{am}$%��
���� ���⿼���˹���������ˮ��Ӧ������ʵ���Լ��ⶨ���ʵ�����������ʵ�飬��Ŀ��Ϊ�ۺϣ��ѶȲ���ע�����ʵ�鷽�������ԭ����

| A�� | ��Һ��pH���������� | |
| B�� | ��������п�۷�Ӧ���������������ͬ | |
| C�� | ������п����Ӧʱ��һ��ʼ��������ʿ� | |
| D�� | ���к�NaOH��Һ�����ʵ���������� |

��1����ͼ1��ԭ���������������ֶ�����Ԫ�ص�һ������ʾ��ͼ��������B��������O��Ԫ�طֱ��Ǣݡ��ۣ���ͼ��Ԫ�ش��ţ���
��2��PCl6-��SF6����������Ľṹ���ֱ���ͼ2��3����PCl6-���������ǣ���ǡ���������λ����ԭ����Pԭ��ֻ��5���۵��ӣ����ڸ�������Pԭ���γ���6�����ۼ�������һ����λ����
���SF6�е����ɸ�Fԭ�ӱ�Clԭ��ȡ���γ�SFxCl6-x ��x��Ϊ0������SFxCl6-x��ͬ��������ΪC��
A��6�� B��7�� C��9�� D��12��
��3��Mn��Fe��Ϊ�������ڹ���Ԫ�أ���Ԫ�صIJ��ֵ����ܣ�I���������������
| Ԫ�� | Mn | Fe | |
| �����ܣ�KJ•mol-1�� | ��1 | 717 | 759 |
| ��2 | 1509 | 1561 | |
| ��3 | 3248 | 2957 | |
Feԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������[Fe��CN��6]3- �е�����CN- ��Cԭ�ӵ��ӻ����������sp��д��һ����CN- ��Ϊ�ȵ�����ĵ��ʷ��ӵĽṹʽN��N��
��1����PM2.5����������ˮ�����Ƴɴ���������
����ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�������
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϣ���֪��H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8kJ•mol-1
C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.5kJ•mol-1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ��C��s��+H2O��g��=CO��g��+H2��g����H=+13l.3kJ•mol-1��
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����a��b��
a��Ca��OH��2 b��Na2CO3 c��CaCl2d��NaHSO3
��3������β����NOx��CO�����ɼ�ת��
����֪����������NO�ķ�ӦΪ��N2��g��+O2��g��?2NO��g����H��0��1mol������0.8molN2��0.2molO2��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⣬���NOΪ8��10-4mol��������¶��µ�ƽ�ⳣ��K=4��10-6�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ�����¶����ߣ���Ӧ���ʼӿ죬ƽ�����ƣ�
��Ŀǰ��������β��ϵͳ�а�װ��ת�����ɼ���CO��NO����Ⱦ����Ӧ����ʽΪ2CO+2NO$\frac{\underline{\;����\;}}{\;}$2CO2+N2��
| A�� | �����Ƕ���ֲ��������������Ҫ��Դ����֬�Dz���������ߵ�Ӫ������ | |
| B�� | ��ʯȼ�ϵĴ���ʹ���Dz���PM2.5����Ҫԭ��֮һ | |
| C�� | ̼�����������Ҳ���մ�������ϴ�����þߵ����� | |
| D�� | ��ͭ���ҹ�ʹ������ĺϽ���ϣ�Ŀǰ������ʹ�������ĺϽ���������Ͻ� |
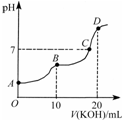
| A�� | KHC2O4��Һ�������� | |
| B�� | B��ʱ��c��HC2O4-����c��K+����c��H+����c��OH-�� | |
| C�� | C��ʱ��c��HC2O4-��+c��C2O42-��+c��H2C2O4����c��K+����c��HC2O4-��+2c��C2O42-��+c��H2C2O4�� | |
| D�� | D��ʱ��c��H+��+c��HC2O4-��+c��H2C2O4��=c��OH-�� |
 ��
��