��Ŀ����
ͨ������£�����ǻ�����ͬһ��̼ԭ���ϵķ��ӽṹ�Dz��ȶ��ģ������Զ�ʧˮ����̼��˫���Ľṹ�� ����ͼ��ʾ�Ǿ��ֻ������ת���ϵ��
����ͼ��ʾ�Ǿ��ֻ������ת���ϵ��

��1��������ٵĽṹ��ʽ�� ����������������Ӧ�������� ��
��2��������߽ṹ��ʽ�� ���ɢܡ��ߵ�ˮ�������� ��
��3��������ݸ��߷��Ӽ���ˮ���ɻ�����ᣬ��Ľṹ��ʽ ��
��4��д�����й��̵Ļ�ѧ��Ӧ����ʽ��
�ݡ��ޣ� ��
��+�ڡ�� ��
 ����ͼ��ʾ�Ǿ��ֻ������ת���ϵ��
����ͼ��ʾ�Ǿ��ֻ������ת���ϵ��
��1��������ٵĽṹ��ʽ��
��2��������߽ṹ��ʽ��
��3��������ݸ��߷��Ӽ���ˮ���ɻ�����ᣬ��Ľṹ��ʽ
��4��д�����й��̵Ļ�ѧ��Ӧ����ʽ��
�ݡ��ޣ�
��+�ڡ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
����������������Ӧ�õ��ۣ��ɢ۵Ľṹ��֪����Ϊ ���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ
���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ ��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ
��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ ����Ϊ
����Ϊ ����Ϊ
����Ϊ �����״��뱽���ᷴӦ�õ���Ϊ
�����״��뱽���ᷴӦ�õ���Ϊ ���ݴ˽��
���ݴ˽��
 ���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ
���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ ��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ
��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ ����Ϊ
����Ϊ ����Ϊ
����Ϊ �����״��뱽���ᷴӦ�õ���Ϊ
�����״��뱽���ᷴӦ�õ���Ϊ ���ݴ˽��
���ݴ˽�����
�⣺����������Ӧ�õ��ۣ��ɢ۵Ľṹ��֪����Ϊ ���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ
���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ ��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ
��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ ����Ϊ
����Ϊ ����Ϊ
����Ϊ �����״��뱽���ᷴӦ�õ���Ϊ
�����״��뱽���ᷴӦ�õ���Ϊ ��
��
��1���ɢ١��ۿ�֪����Ϊ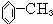 ����������Ӧ���������·���ȡ����Ӧ��
����������Ӧ���������·���ȡ����Ӧ��
�ʴ�Ϊ��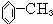 �����գ�
�����գ�
��2��������ķ�����֪��Ϊ ���ɢܡ��ߵ�ˮ��������������������Һ�У�
���ɢܡ��ߵ�ˮ��������������������Һ�У�
�ʴ�Ϊ�� ������������Һ��
������������Һ��
��3����Ϊ ����Ϊ�����ᣬ���߷����û���Ӧ���ɱ����ᱽ�������ṹʽΪ
����Ϊ�����ᣬ���߷����û���Ӧ���ɱ����ᱽ�������ṹʽΪ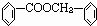 ��
��
�ʴ�Ϊ��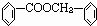 ��
��
��4���ݡ��Ļ�ѧ��Ӧ����ʽΪ����ʽΪ2C6H5CH2OH+O2
2C6H5CHO+2H2O��
��+�ڡ���Ļ�ѧ��Ӧ����ʽΪ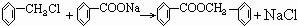 ��
��
�ʴ�Ϊ��2C6H5CH2OH+O2
2C6H5CHO+2H2O��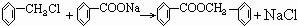 ��
��
 ���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ
���ױ��������ڹ��������·������ϵ�ȡ����Ӧ����Ϣݣ����״����Ľṹ��֪��Ϊ ��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ
��������̼�����Ʒ�Ӧ�õ��࣬�ʢߺ����Ȼ������Ϊ ����Ϊ
����Ϊ ����Ϊ
����Ϊ �����״��뱽���ᷴӦ�õ���Ϊ
�����״��뱽���ᷴӦ�õ���Ϊ ��
����1���ɢ١��ۿ�֪����Ϊ
 ����������Ӧ���������·���ȡ����Ӧ��
����������Ӧ���������·���ȡ����Ӧ���ʴ�Ϊ��
 �����գ�
�����գ���2��������ķ�����֪��Ϊ
 ���ɢܡ��ߵ�ˮ��������������������Һ�У�
���ɢܡ��ߵ�ˮ��������������������Һ�У��ʴ�Ϊ��
 ������������Һ��
������������Һ����3����Ϊ
 ����Ϊ�����ᣬ���߷����û���Ӧ���ɱ����ᱽ�������ṹʽΪ
����Ϊ�����ᣬ���߷����û���Ӧ���ɱ����ᱽ�������ṹʽΪ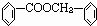 ��
���ʴ�Ϊ��
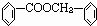 ��
����4���ݡ��Ļ�ѧ��Ӧ����ʽΪ����ʽΪ2C6H5CH2OH+O2
| Cu/Ag |
| �� |
��+�ڡ���Ļ�ѧ��Ӧ����ʽΪ
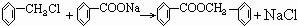 ��
���ʴ�Ϊ��2C6H5CH2OH+O2
| Cu/Ag |
| �� |
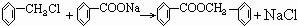 ��
��
���������⿼���л���Ľṹ�����ʣ���Ŀ�Ѷ��еȣ�ע����ݢ١��۵ķ�Ӧ���ƶϢ٣��������ʵ����ʿ��ƶ��������ʣ���ȷ�ƶϸ����ʵĽṹ�ǽ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��������һ����ȷ���ǣ�������
| A��H2O��D2O��T2O��Ϊͬλ�� |
| B��pH=1����Һ�У�K+��Na+��NO3-��S2O32-���ܴ������� |
| C��Ԫ�����ڱ��д�������ͬ����Ԫ�ص��⻯����ۡ��е��������� |
| D����Ȳ�����������ֱ���������Ӧ��������������������ͬ�����ɲ�ͬ���� |
��ʾ���б仯�Ļ�ѧ������ȷ���ǣ�������
| A��NaHCO3��ˮ�⣺HCO3-+H2O?H3O++CO32- | ||||
| B��1L 0.5mol?L-1ϡ������1L 1mol?L-1����������Һ��Ӧ�ų�57.3kJ��������H2SO4��aq��+2NaOH��aq���TNa2SO4��aq��+2H2O��1������H=-57.3 kJ/mol | ||||
| C������ȼ�ϵ�صĸ�����Ӧʽ��O2+2H2O+4e-�T4OH- | ||||
D���Զ��Ե缫���KCl��Һ��2Cl-+2H2O
|
ϡ���ǹ�ҵζ������Сƽ˵�����ж���ʯ�ͣ�������ϡ������ϡ��Ԫ���棨Ce����Ҫ�����ڶ���ʯ�У��������ڿ������������䰵������ʱȼ�գ���ˮ�ܿ췴Ӧ����֪���泣���Ļ��ϼ�Ϊ+3��+4�������ԣ�Ce4+��Fe3+������˵����ȷ���ǣ�������
| A����֪Ceԭ��������58������Ϊ��ϵԪ�� | ||||||||
B�����������ȶ��ĺ�
| ||||||||
| C����Ce��SO4��2��Һ��������������Һ��Ӧ�������ӷ���ʽΪ��Ce4++2Fe2+=Ce3++2Fe3+ | ||||||||
| D�������������Ļ�ѧ����ʽ�ɱ�ʾΪ��Ce+4HI=CeI4+2H2�� |



