��Ŀ����
4����1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ�����ˮ���ų�����642kJ��25��ʱ����N2H4��ȫȼ�յ��Ȼ�ѧ����ʽ�ǣ�N2H4��l��+O2��g��=N2��g��+2H2O��1����H=-642.0kJ/mol����2���ڸ�����һ����̼�ɽ���������ԭΪ������
��C��s��+O2��g��=CO2��g����H1=-393.5kJ•mol
��CO2��g��+C��s��=2CO��g����H2=+172.5kJ•mol
��S��s��+O2��g��=SO2��g����H3=-296.0kJ•mol
��CO��SO2��Ӧ���Ȼ�ѧ����ʽ��2CO��g��+SO2��g��=CO2��g��+S��s����H=-270kJ/mol��
��3�������������Һ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪNa2S2O3+H2SO4�TNa2SO4+SO2��+S��+H2O�����и���ʵ�������ȳ��ֻ��ǵ���D������ĸ���ţ���
| ʵ�� | ��Ӧ�¶�/�� | Na2S2O3��Һ | ϡH2SO4 | H2O | ||
| V/mL | c/��mol•L-1�� | V/mL | c/��mol•L-1�� | V/mL | ||
| A | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| B | 25 | 5 | 0.2 | 5 | 0.2 | 10 |
| C | 35 | 5 | 0.1 | 10 | 0.1 | 5 |
| D | 35 | 5 | 0.2 | 5 | 0.2 | 10 |
���� ��1�������Ȼ�ѧ����ʽ��ע��������д��
��2�����ø�˹���ɢ�-��-�۵ã�2CO��g��+SO2��g��=CO2��g��+S��s����H=-270kJ/mol
��3��ʵ��A��ʵ��B��ʵ��B��Na2S2O3��H2SO4��
Ũ�ȶ���Ӧ���ʴ�ʵ��C��ʵ��
D���¶ȱ�ʵ��A��ʵ��B�ߣ���Ӧ
���ʴ�ʵ��C��ʵ��
D�У�ʵ��D��Na2S2O3��H2SO4��
Ũ�ȶ���Ӧ���ʴ�����ʵ��
D��Ӧ����������ȳ��ֻ��ǣ�
��4��H+��aq��+OH-��aq���TH2O��1����H=-57.3kJ/mol
1
0.25L0.10mol/L Q
�б���Ϊ1����H=0.25L0.10mol/L��Q������Q=cm��t=4.2��10-3kJ/��g•�棩��500mL��1.0g/mL����t
����t=0.68��
��� 
���� ע�⣺�к�����ϡ���ᡢ���к����� 1 molˮ�ķ�Ӧ�ȣ���1 molˮΪ�����м��㣮

��ϰ��ϵ�д�
 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�
�����Ŀ
10����ѧ�����������ϵ������˵������ȷ���ǣ�������
| A�� | Na2SiO3ˮ��Һ�׳�ˮ������������ľ�ķ���� | |
| B�� | Fe3O4�׳����죬��������ɫͿ�� | |
| C�� | Na2CO3�׳ƴ������Ϊ���첣����ԭ�� | |
| D�� | KAl��SO4��2•12H2O�׳�����������Ϊ��ˮ�� |
19����֪W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�W����̬�⻯����ȶ��Դ���Z����̬�⻯���ȶ��ԣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԣ�����˵����ȷ���ǣ�������
| A�� | X��Y��Z��W��ԭ�Ӱ뾶���μ�С | |
| B�� | W��X�γɵĻ�������ֻ�����Ӽ� | |
| C�� | W�����������һ����Z�ĺ����������ǿ | |
| D�� | ��W��Y��ԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3 |
9�����з�Ӧ�������ӷ���ʽCO32-+Ba2+�TBaCO3����ʾ���У�������
| A�� | BaCl2��K2CO3��Һ��Ӧ | B�� | CO2��Ba��OH��2��Һ��Ӧ | ||
| C�� | Ba��NO3��2��Na2CO3��Һ��Ӧ | D�� | Ba��OH��2������NaHCO3��Һ��Ӧ |
16�������£����и���������ָ����Һ��һ���ܴ���������ǣ�������
| A�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012����Һ�У�Mg2+��Al3+��NO3-��Cl- | |
| B�� | ʹpH��ֽ����ɫ����Һ�У�NH4+��NO3-��SO42-��Na+ | |
| C�� | ��ˮ�����c��H+��=1��10-14mol•L-1����Һ�У�Mg2+��K+��Cl-��NO3- | |
| D�� | pH=0����Һ�У�K+��Fe3+��SO42-��SCN- |
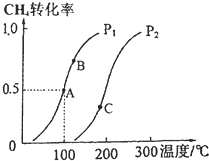 ������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪ��CH4��g��+H2O��g��?CO��g��+3H2��g����
������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪ��CH4��g��+H2O��g��?CO��g��+3H2��g����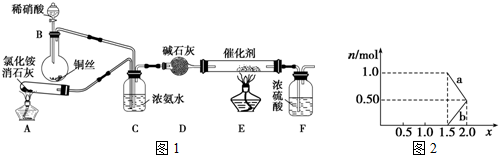
 �����̬ԭ����7��������ͬ�ĵ��ӣ�
�����̬ԭ����7��������ͬ�ĵ��ӣ�
 �����������У�±��Ԫ�أ�F��Cl��Br��I���ĵ��ʼ���������;�㷺��
�����������У�±��Ԫ�أ�F��Cl��Br��I���ĵ��ʼ���������;�㷺��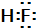 ����HF��HCl��������з����HF�ķ�����������
����HF��HCl��������з����HF�ķ����������� ��
��