��Ŀ����
15��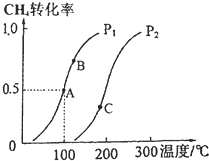 ������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪ��CH4��g��+H2O��g��?CO��g��+3H2��g����
������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪ��CH4��g��+H2O��g��?CO��g��+3H2��g������1��������ˮ������Ӧ����������Ԫ����C��̼���������ɱ�״����35.84L�ϳ���ʱת�Ƶ��ӵ����ʵ�����2.4mol��
��2����2mol CH4��5mol H2O��g��ͨ���ݻ�Ϊ100L�ķ�Ӧ�ң�CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
�ٴﵽA�������ʱ��Ϊ5min����v��H2��=0.006mol•L-1•min-1��100��ʱƽ�ⳣ��K=6.75��10-4mol2•L-2��
��ͼ�е�P1��P2�����������������=������A��B��C�����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��KC��KB��KA��
��3���ϳ������ںϳɰ���ʱ���ȥCO��������ӦCO��g��+H2O��g��?CO2��g��+H2��g����H��0�����д�ʩ����ʹ$\frac{n��C{O}_{2}��}{n��{H}_{2}O��}$�����AC��ѡ���ţ���
A�������¶� B�����º����³���He��g��
C����H2����ϵ�з��� D����ͨ��һ������ˮ����
����̼�����Һ�������ɵ�CO2��������pH=10��̼�����Һ��ˮ�����OH-�����ʵ���Ũ��Ϊ1��10-4mol•L-1�������£�0.1mol•L-1KHCO3��ҺpH��8������Һ��c��H2CO3����c��CO32-�������������=����������
���� ��1���÷�Ӧ��CԪ�ػ��ϼ���-4�۱�Ϊ+2�ۡ�HԪ�ػ��ϼ���+1�۱�Ϊ0�ۣ�ʧ���ӻ��ϼ����ߵ�Ԫ�ر������������ɱ�״����35.84L�ϳ���ʱ������n��CO��=$\frac{35.84L}{22.4L/mol}$=0.4mol������CԪ�ػ��ϼ۱仯ȷ��ת�Ƶ��ӵ����ʵ�����
��2���ٸ���v=$\frac{\frac{��n}{V}}{��t}$�������ķ�Ӧ���ʣ��ٸ���ͬһ���淴Ӧ��ͬһʱ����ڸ����ʵķ�Ӧ����֮�ȵ����������֮�ȼ��������ķ�Ӧ���ʣ�
��ѧƽ�ⳣ��K=$\frac{c��CO����{c}^{3}��{H}_{2}��}{c��C{H}_{4}����c��{H}_{2}O��}$��
����ͬ���������£�����ѹǿ��ƽ�����淴Ӧ�����ƶ��������ת��Ϊ��С����ѧƽ�ⳣ��ֻ���¶��йأ������¶ȣ������ת��������������Ӧ�����ȷ�Ӧ���¶�Խ�ߣ���ѧƽ�ⳣ��Խ��
��3����ʹ$\frac{n��C{O}_{2}��}{n��{H}_{2}O��}$���÷�ӦӦ��������Ӧ�����ƶ�������ͨ������ˮ���������ʵ���ʵ�֣�
ˮ�����OH-�����ʵ���Ũ��=$\frac{1{0}^{-14}}{1{0}^{-pH}}$�������£�0.1mol•L-1 KHCO3��ҺpH��8��˵��̼�������ˮ��̶ȴ��ڵ���̶ȣ�
��� �⣺��1���÷�Ӧ��CԪ�ػ��ϼ���-4�۱�Ϊ+2�ۡ�HԪ�ػ��ϼ���+1�۱�Ϊ0�ۣ�ʧ���ӻ��ϼ����ߵ�Ԫ�ر�����������CԪ�ر������������ɱ�״����35.84L�ϳ���ʱ�����ɣ�CO��=$\frac{35.84L}{22.4L/mol}$=0.4mol����ת�Ƶ��ӵ����ʵ���=0.4mol��[2-��-4��]=2.4mol��
�ʴ�Ϊ��C��̼����2.4mol��
��2���ټ���ķ�Ӧ����=$\frac{\frac{2mol��0.5}{100L}}{5min}$=0.002mol/��L��min����ͬһ���淴Ӧ��ͬһʱ����ڸ����ʵķ�Ӧ����֮�ȵ����������֮�ȣ��������ķ�Ӧ����=3��0.002mol/��L��min��=0.006mol/��L��min����
100��ʱ��c��CH4��=$\frac{2mol����1-0.5��}{100L}$=0.01mol/L��c��H2O��=$\frac{5mol-2mol����1-0.5��}{100L}$=0.04mol/L��
c��CO��=$\frac{2mol��0.5}{100L}$=0.01mol/L��c��H2��=3c��CO��=0.03mol/L����ѧƽ�ⳣ��K=$\frac{c��CO����{c}^{3}��{H}_{2}��}{c��C{H}_{4}����c��{H}_{2}O��}$=$\frac{0.01����0.03��3}{0.01��0.04}$=6.75��10-4��
�ʴ�Ϊ��0.006mol•L-1•min-1��6.75��10-4 mol2•L-2��
�ڸ���ͼ��֪������ѹǿ��ƽ�����淴Ӧ�����ƶ���������ת���ʽ��ͣ�����P1��P2��
�����¶ȣ������ת��Ϊ����˵������Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�ƽ�ⳣ��Խ������KC��KB�� KA��
�ʴ�Ϊ������KC��KB�� KA��
��3��A�������¶ȣ�ƽ��������Ӧ�����ƶ�������ʹ$\frac{n��C{O}_{2}��}{n��{H}_{2}O��}$������ȷ��
B�����º����³���He��g������Ӧ���������Ũ�Ȳ��䣬��ƽ�ⲻһ������$\frac{n��C{O}_{2}��}{n��{H}_{2}O��}$���䣬�ʴ���
C����H2����ϵ�з��룬ƽ��������Ӧ�����ƶ�����$\frac{n��C{O}_{2}��}{n��{H}_{2}O��}$������ȷ��
D����ͨ��һ������ˮ������ƽ��������Ӧ�����ƶ�����ˮת����������ԶԶС�ڼ������������������ʹ$\frac{n��C{O}_{2}��}{n��{H}_{2}O��}$��С���ʴ���
��ѡ��AC��
����̼�����Һ�������ɵ�CO2��������pH=10��̼�����Һ��ˮ�����c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-10}}$mol/L=1��10-4 mol•L-1�������£�0.1mol•L-1 KHCO3��ҺpH��8������Һ��̼���������ˮ��̶ȴ��ڵ���̶ȣ�����c��H2CO3����c��CO32-�����ʴ�Ϊ��AC�� 1��10-4 mol•L-1������
���� ���⿼����ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ����ѧƽ���ƶ���Ӱ�����ء�����ˮ���֪ʶ�㣬�����ͼ�����¶ȡ�ѹǿ�¶ȷ�Ӧ�ȡ�ƽ���ƶ�����ע�⻯ѧƽ�ⳣ��ֻ���¶��йأ�ע�⣨3����BDѡ������ѹ�����³���He����ƽ��ᷢ���ƶ���������������ͨ��He����ƽ�ⲻ�ƶ���Ϊ�״��㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���NH3���ۡ��е��VA������Ԫ���⻯����ۡ��е��
��С���ӵĴ���������Ժ�ˮ������Ȼ���
�����ǻ���������ۡ��е�ȶ��ǻ���������ۡ��е��
��ˮ���Ӹ�����Ҳ���ȶ���ˮ���ӽ���������ȶ���
| A�� | �٢ڢۢ� | B�� | �ܢ� | C�� | �ۢܢ� | D�� | �٢ڢ� |

���ٶ�����̼���ŷ���һ����Ҫ���⣮�о�������C02��������ɺϳɵ�̼����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H���ֻ�ѧ���ļ��������ʾ
| ��ѧ�� | C=0 | H-H | C-C | C-H | 0-H | C-0 |
| ����/kJ•mol-1 | 803 | 436 | 332 | 409 | 463 | 326 |
��2����1.0L�����ܱ�������Ͷ��lmolC02��2.75molH2�����÷�Ӧ��ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ���������ʵ�����ͼ��ʾ��
��ѹǿΪP2���¶�Ϊ512Kʱ�����������Ͷ�� lmol CO2��0.5mol H2��2mol CH3OH��0.6mol H20ʱ��ƽ�����淴Ӧ�����ƶ���
��3����CH3OH��������02����20%����KOH��ҺΪ ԭ�ϣ���ʯīΪ�缫��ֱ�ӹ���ȼ�ϵ�أ���õ�صĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O���ø� ��ص��500mL 1mol/L CuSO4��Һ����ȼ�ϵ�����ı����56L����ʱ��������������������������������ʵ���1mol��
��SO2Ҳ��һ�ִ�����Ⱦ������ú�ˮ������SO2�ķ�������ˮ����Ҫ���ӵĺ������£�
| �ɷ� | ����/��mg/L�� | �ɷ� | ������mg/L�� |
| Cl- | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3-�� | 142 |
| SO42- | 2560 | Mg2+ | 1272 |
��֪Ksp[Mg��OH��2]=1.2��l0-11 Ksp[Ca��OH��2]=5.5��10-6
��2���������ķ�����S02�ĺ�������ͨ�����������ⶨ���õ����Լ��У�0��l mol•L�ĵ����Һ��������Һ�����Ѵ�����ķ�����ÿ����aL������£�������ͨ�뵽lOOmL���е��۵ĵ��Һ�У�t min�ﵽ�ζ��յ㣮�ζ��յ������Ϊ��Һ��ɫ��ȥ���Ұ���Ӳ��ָ���ɫ����ô�������SO2�ĺ���$\frac{0.224}{at}$���ú�a��t��ʽ�ӱ�ʾ��
| A�� | ��Ӧ�����У�������ѹǿ�����SiCl4��ת���� | |
| B�� | ����Ӧ��ʼʱSiCl4Ϊ1mol����ﵽƽ��ʱ����������ΪQkJ | |
| C�� | ��Ӧ��4minʱ����HCl��Ũ��Ϊ0.12mol•L-1����H2�ķ�Ӧ����Ϊ��0.015mol/��L•min�� | |
| D�� | ����Ӧ��������Ϊ0.025QkJʱ�����ɵ�HClͨ��100mL1mol•L-1��NaOH��Һǡ�÷�Ӧ |
��1����֪̼��������Ӧ�ڲ�ͬ�¶���ƽ�ⳣ���Ķ���ֵ��lgK�����±���
| ������Ӧʽ | 1gK | ||
| 700K | 900K | 1200K | |
| C��s��+H2O��g��=CO��g��+H2��g�� | -2.64 | -0.39 | 1.58 |
| C��s��+2H2O��g��=CO2��g��+2H2��g�� | -1.67 | -0.03 | 1.44 |
��2��ҵ�Ϻϳɼ״��ķ�ӦΪ��CO+2H2?CH3OH����֪��H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ/mol��-283.0kJ/mol��-726.5kJ/mol����CH3OH��l������ȫȼ������CO��Һ̬H2O���Ȼ�ѧ��Ӧ����ʽΪCH3OH��l��+O2��g��=CO��g��+2H2O��l������H=-443.5kJ/mol��
��3����һ���¶ȡ�ѹǿ�ʹ������£���ҵ����H2CO��CO�ϳ�CH3OCH3��
3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H=-246.4KJ•mol-1
����һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ�⣬ֻ�ı�һ��������ͬʱ��߷�Ӧ���ʺ�CO��ת���ʵ���cd������ĸ��ţ���
a�������¶� b��������� c����С������� d������H2��Ũ�� e������CO��Ũ��
����һ����ɱ���ܱ������г���3molH2��3molCO��1molCH3OCH3��1molCO2����һ���¶Ⱥ�ѹǿ�·���������Ӧ����һ��ʱ��ﵽƽ�⣬���ƽ��ʱ���������ܶ���ͬ��ͬѹ����ʼ��1.6������Ӧ��ʼʱ�����淴Ӧ���ʵĴ�С��v����v�棨�������������=������ƽ��ʱCO�����ʵ�������Ϊ15%��
��4��һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���amol•L-1�Ĵ�����bmol•L-1��Ba��OH��2��Һ�������ϣ���ַ�Ӧ����Һ�д���2c��Ba2+��=c��CH3COO-������û����Һ�д���ĵ��볣��Ka=$\frac{2b}{a-2b}$��10-7 mol•L-1���ú�a��b�Ĵ���ʽ��ʾ������仯���Բ��ƣ���
��2���ڸ�����һ����̼�ɽ���������ԭΪ������
��C��s��+O2��g��=CO2��g����H1=-393.5kJ•mol
��CO2��g��+C��s��=2CO��g����H2=+172.5kJ•mol
��S��s��+O2��g��=SO2��g����H3=-296.0kJ•mol
��CO��SO2��Ӧ���Ȼ�ѧ����ʽ��2CO��g��+SO2��g��=CO2��g��+S��s����H=-270kJ/mol��
��3�������������Һ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪNa2S2O3+H2SO4�TNa2SO4+SO2��+S��+H2O�����и���ʵ�������ȳ��ֻ��ǵ���D������ĸ���ţ���
| ʵ�� | ��Ӧ�¶�/�� | Na2S2O3��Һ | ϡH2SO4 | H2O | ||
| V/mL | c/��mol•L-1�� | V/mL | c/��mol•L-1�� | V/mL | ||
| A | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| B | 25 | 5 | 0.2 | 5 | 0.2 | 10 |
| C | 35 | 5 | 0.1 | 10 | 0.1 | 5 |
| D | 35 | 5 | 0.2 | 5 | 0.2 | 10 |
| A�� | ����������Һ�У�Na+��K+��MnO4-��NO3- | |
| B�� | pH=13����Һ�У�Na+��K+��SO42-��HCO3- | |
| C�� | 0.1 mol•L-1 NaClO��Һ�У�K+��Na+��NO3-��I- | |
| D�� | 0.1 mol•L-1 FeCl3��Һ�У�Na+��NH4+��SCN-��SO42- |

