��Ŀ����
20��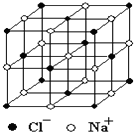 ������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl�ľ�����ͼ��ʾ�����ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ���еIJⶨ���������岽�����£��ٽ�NaCl������ϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У����õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV mL���ش��������⣺
������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl�ľ�����ͼ��ʾ�����ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ���еIJⶨ���������岽�����£��ٽ�NaCl������ϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У����õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV mL���ش��������⣺��1�������������A���������ƿ�����������ƣ���
��2������Ϊ�������������ʽ�����ʽ����ʽ�����ζ��ܣ�ԭ�������ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ��ܽ⣩��
��3���ܷ��ý�ͷ�ιܴ��沽����еĵζ��ܲ��ܣ���ԭ����ʵ������Ҫȷ��ȡ���������
��4����X-����������NaCl����������Na+��Cl-�ĺ˼��Ϊacm������������������õİ����ӵ���������ѧ����ʽΪNA=$\frac{29.25V}{m{a}^{3}}$��
���� ��1������ƿΪ�����������ܹ���ȷ�IJⶨ�����
��2����Ӧ����ʽ�ζ���ʢװ�����ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�
��3��ʵ������Ҫȷ��ȡ�����������˲����ý�ͷ�ιܴ���ζ��ܣ�
��4������NaCl���ܶȺ;������������һ��NaCl����������������1molNaCl��������Ħ�������Ͱ����ӵ������Ĺ�ϵ���㰢���ӵ�������
��� �⣺��1����������Ϊ����ƿ������һ����������������Բ�������������������ƿ��
�ʴ�Ϊ������ƿ��
��2�������и�ʴ�ԣ���ʴ��ʽ�ζ����е���Ƥ�ܣ�ֻ������ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ�����ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ��ܽ⣩��
��3��ʵ������Ҫȷ��ȡ�����������˲����ý�ͷ�ιܴ���ζ��ܣ�
�ʴ�Ϊ��ʵ������Ҫȷ��ȡ���������
��4��NaCl���ܶ�Ϊ$\frac{m}{V}$g/cm3��
NaCl�����������2a��3cm3��
��NaCl������������$\frac{m}{V}$g/cm3����2a��3cm3=$\frac{m}{V}$g/cm3����2a��3g��
һ��NaCl������4����NaCl����
��ÿ����NaCl��������Ϊ$\frac{M��NaCl��}{NA}$��
$\frac{m}{V}$g/cm3����2a��3=4��$\frac{58.5}{NA}$��
��NA=$\frac{58.5V}{2m{a}^{3}}$��
�ʴ�Ϊ��$\frac{29.25V}{m{a}^{3}}$��
���� ���⿼�龧���Ľṹ�����Ͱ����ӵ������IJⶨ����Ŀ���ѣ�ע�����1molNaCl��������Ħ�������Ͱ����ӵ������Ĺ�ϵ���㰢���ӵ�������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��NaClO��Һ��ͨ�����SO2��ClO-+SO2+H2O�THClO+HSO3- | |
| B�� | ��AlCl3��Һ��Ͷ�����Na��Al3++4Na+2H2O�TAlO2-+4Na++2H2�� | |
| C�� | ��FeBr2��Һ��ͨ������Cl2��2Fe2++4Br-+3Cl2�T2Fe3++2Br2+6Cl- | |
| D�� | ��������Һ�еμ�Ba��OH��2��Һ��ǡ��ʹSO42-��ȫ������2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4 |
��1������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�У�CuSO4+Zn�TZnSO4+Cu��Zn+H2SO4�TZnSO4+H2����
��2��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ�е�ʵ�飮�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䣮
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4mol•L-1 H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�ڸ�ͬѧ���ó��Ľ���Ϊ����������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ����һ������CuSO4�����ɵĵ���Cu�������Zn�ı��棬������Zn��H2SO4��Һ�ĽӴ������ʹ��Ӧ�����½���
��1��д���ζ������з�����Ӧ�����ӷ���ʽ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O
��2�����Ʊ���Һ500.00ml�����ƹ������õ�������������ƽ��ҩ�ף��ձ������������Ҫ500mL����ƿ����ͷ�ι�
��3����KMnO4�ζ�������Һ����Ҫѡָʾ��������Ҫ������ָʾ�����ƣ�������Ҫ���ϡ��ޡ�KMnO4��Һ���ڲ���������������ת�ζ��ܵIJ�����������������ƿ���յ�ʱ���������һ��KMnO4��Һ����ʱ����ҺΪ�Ϻ�ɫ���Ұ�����ڲ���ɫ
��4����¼�������£�
| �ζ����� | ������Һ���/ml | KMnO4��Һ���/ml | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
��5�����в����ᵼ�²ⶨ����Ũ��ƫ�ߵ���AC
A���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ
B�����Ʊ���Һʱ���ӿ̶���
C��δ�ñ�Һ��ϴ�ζ���
D���ζ�ǰʢ�Ų�����Һ����ƿ������ˮϴ����û�и��
| A�� | HNO3��Һ����OH- | |
| B�� | �����£��κ����ʵ�ˮ��Һ�ж���H+��OH-����KW=10-14 | |
| C�� | NaCl��Һ�м���OH-Ҳ��H+ | |
| D�� | ����������Һ����H+ |
| MnO2 | �����Թ���� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ���� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | �� | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��2��ʵ���������������Ĵ�Ч��������ı�����йأ�
��3��ijͬѧ��10mL H2O2 ��Һ�м���һ�����Ķ������̣��ų�������������״�����뷴Ӧʱ��Ĺ�ϵ��ͼ1��ʾ����A��B��C��������ʾ�ķ�Ӧ������������C��
��ij��Ӧ�����Ϊ5L�ĺ����ܱ������н��У���0-3�����ڸ����ʵ����ı仯�����ͼ2��ʾ��A��B��C��Ϊ���壬��A��������ɫ����

��4���÷�Ӧ�Ļ�ѧ����ʽΪ2A+B?2C��
��5����Ӧ��ʼ��2����ʱ��B��ƽ����Ӧ����Ϊ0.1mol/��L•min����
��6����˵���÷�Ӧ�Ѵﵽƽ��״̬����cd��
a��v��A��=2v��B��
b�������ڸ����ʵ����ʵ������
c��v����A��=v����C��
d���������������ɫ���ֲ���
��7����ͼ���ƽ��ʱA���������37.5%��
 ��1���£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬���������ȼ�ϣ���֪��101kPa��320g N2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�յ��Ȼ�ѧ����ʽ�ǣ�N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-62.4KJ/mol
��1���£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬���������ȼ�ϣ���֪��101kPa��320g N2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�յ��Ȼ�ѧ����ʽ�ǣ�N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-62.4KJ/mol