��Ŀ����
9�� ��1���£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬���������ȼ�ϣ���֪��101kPa��320g N2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�յ��Ȼ�ѧ����ʽ�ǣ�N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-62.4KJ/mol
��1���£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬���������ȼ�ϣ���֪��101kPa��320g N2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�յ��Ȼ�ѧ����ʽ�ǣ�N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-62.4KJ/mol��2����-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20��30%��KOH��Һ����-����ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ��2H2O+O2+4e-=4OH-�������ĵ缫��Ӧʽ��N2H4+4OH--4e-=4H2O+N2����
��3����ͼ��һ���绯ѧ����ʾ��ͼ��
����ʹ����-����ȼ�ϵ����Ϊ�����̵ĵ�Դ��ͭƬ�����仯128g������-����ȼ�ϵ�����������ı�״���µĿ���112L������������������������Ϊ20%����
��4����ͳ�Ʊ��µķ�������NaClO����NH3�Ƶ��µ�ϡ��Һ���÷�Ӧ�����ӷ���ʽ��ClO-+2NH3=N2H4+Cl-+H2O��
���� ��1�������ºͷ�Ӧ�ȵĹ�ϵ�������ȼ���ȣ���д������Ӧ���Ȼ�ѧ����ʽ��
��2��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ���ڼ��Ի����µķ�ӦʽΪ��N2H4+4OH--4e-=4H2O+N2���������������������õ��ӵĻ�ԭ��Ӧ���ڼ��Ի����£��缫��ӦʽΪ��2H2O+O2+4e-=4OH-��
��3������ת�Ƶ�����ȼ�����Ҫ�����������
��4��NaClO����NH3�����Ƶ��£��������Ȼ��ƣ�
��� �⣺��1����320g�µ����ʵ���Ϊ10mol��10molN2H4����������ȫȼ�����ɵ����ų�����624kJ���������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-62.4KJ/mol��
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-62.4KJ/mol��
��2��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ���ڼ��Ի����µķ�ӦʽΪ��N2H4+4OH--4e-=4H2O+N2���������������������õ��ӵĻ�ԭ��Ӧ���ڼ��Ի����£��缫��ӦʽΪ��2H2O+O2+4e-=4OH-���ʴ�Ϊ��2H2O+O2+4e-=4OH-��N2H4+4OH--4e-=4H2O+N2����
��3���������ͭ��Һʱ��ͭ�缫��ͭʧ���ӷ���������Ӧ����ͭƬ�������仯128g��ʧȥ���ӵ����ʵ���=$\frac{128g}{64g/mol}$��1mol��������-2����Ԫ�صõ�4mol���ӣ�����Ҫ���������=$\frac{\frac{4mol}{4}��22.4L/mol}{20%}$=112L���ʴ�Ϊ��112��
��4��NaClO����NH3�����Ƶ��µ����ӷ���ʽΪ��ClO-+2NH3=N2H4+Cl-+H2O��
�ʴ�Ϊ��ClO-+2NH3=N2H4+Cl-+H2O��
���� ������һ���й��Ȼ�ѧ�͵绯ѧ֪ʶ���ۺϿ���֪ʶ��Ŀ��Ҫ��ѧ�����з����ͽ��������������ѶȲ���

| A�� | X��Y��Z����Ԫ�ؿ��γɻ�����X3YZ4 | |
| B�� | X��Y��Ԫ���γɵĻ�����ֻ��Ϊ���ӻ����� | |
| C�� | �⻯����۷е㣺Y��Z | |
| D�� | �⻯����ȶ��ԣ�Y��Z |
| A�� | v��O2��=0.01 mol/��L•s�� | B�� | v��NO��=0.08 mol/��L•s�� | ||
| C�� | v��H2O��=0.03 mol/��L•s�� | D�� | v��NH3��=0.002 mol/��L•s�� |
| A�� | �ơ�þ�����Ļ�ԭ�����μ��� | |
| B�� | NaOH��Mg��OH��2��Al��OH��3����������ǿ | |
| C�� | HCl��HBr��HI���ȶ���������ǿ | |
| D�� | HClO4��H2SO4��H3PO4������������ǿ |
| A�� | ϡ���� | B�� | ��ˮ | C�� | KSCN��Һ | D�� | ���Ը������ |
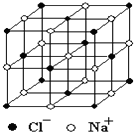 ������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl�ľ�����ͼ��ʾ�����ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ���еIJⶨ���������岽�����£��ٽ�NaCl������ϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У����õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV mL���ش��������⣺
������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl�ľ�����ͼ��ʾ�����ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ���еIJⶨ���������岽�����£��ٽ�NaCl������ϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У����õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV mL���ش��������⣺