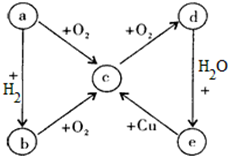
��Ŀ����
15������KMnO4��Һ�������Һ��H2C2O4����Һ��Ӧ������Ũ��Ϊ0.01mol/L����KMnO4��Һ�ζ�δ֪Ũ�ȵIJ��ᣬ��ش��������⣺��1��д���ζ������з�����Ӧ�����ӷ���ʽ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O
��2�����Ʊ���Һ500.00ml�����ƹ������õ�������������ƽ��ҩ�ף��ձ������������Ҫ500mL����ƿ����ͷ�ι�
��3����KMnO4�ζ�������Һ����Ҫѡָʾ��������Ҫ������ָʾ�����ƣ�������Ҫ���ϡ��ޡ�KMnO4��Һ���ڲ���������������ת�ζ��ܵIJ�����������������ƿ���յ�ʱ���������һ��KMnO4��Һ����ʱ����ҺΪ�Ϻ�ɫ���Ұ�����ڲ���ɫ
��4����¼�������£�
| �ζ����� | ������Һ���/ml | KMnO4��Һ���/ml | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
��5�����в����ᵼ�²ⶨ����Ũ��ƫ�ߵ���AC
A���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ
B�����Ʊ���Һʱ���ӿ̶���
C��δ�ñ�Һ��ϴ�ζ���
D���ζ�ǰʢ�Ų�����Һ����ƿ������ˮϴ����û�и��
���� ��1��������ؾ���ǿ�����ԣ��ܰѲ��������ɶ�����̼����������ԭ�ɶ��������ӣ�
��2�����ݲ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ���ѡȡʵ��������
��3��KMnO4��Һ����Ϊ�Ϻ�ɫ��������Һ�г����Ϻ�ɫ�����������ڲ�����ɫ���ͱ��������յ㣻�ζ�ʱ��������ת�ζ��ܵIJ������������ֲ�ͣҡ����ƿ������Ӧ��ע������ƿ����Һ��ɫ�仯��
��4�����жϵζ����ݵ���Ч�ԣ�Ȼ���������ı�Һ��ƽ��������ٸ��ݹ�ϵʽ��2MnO4-��5H2C2O4���������Һ��Ũ�ȣ�
��5������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1��������ؾ���ǿ�����ԣ��Ѳ����е�C��+3��������+4�۵Ķ�����̼��MnԪ�ش�+7�۱仯��+2�۵������ӣ����ڲ����������2��Cԭ�ӣ����Ը�����������ķ�Ӧ����Ϊ 5��2���ʷ�Ӧ�ķ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
��2������0.01mol/L����KMnO4��Һ500.00ml�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�������֪��Ҫʹ�õ�������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��3��KMnO4��Һ����ɫ�����ᷴӦ��ϣ��������һ��KMnO4��Һ����ҺΪ�Ϻ�ɫ���Ұ�����ڲ���ɫ��˵���ζ����յ㣬����Ҫ���ָʾ�����ζ�ʱ��������ת�ζ��ܵIJ���������
�ʴ�Ϊ��KMnO4��Һ����ת�ζ��ܵIJ��������������һ��KMnO4��Һ����ʱ����ҺΪ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��4�����εζ����ı�Һ���Ϊ22.20mL��25.01mL��24.99mL����2���������ϴ���ȥ������2�εζ����ı�Һ��ƽ�����Ϊ25.00mL��������ص����ʵ���Ϊ��0.10mol/L��0.025L=0.0025mol���ɹ�ϵʽ��2MnO4-��5H2C2O4��֪��n��H2C2O4��=$\frac{5}{2}$n��KMnO4��=0.00625mol������Һ��������ʵ���Ũ��Ϊ��$\frac{0.00625mol}{0.025L}$=0.25mol•L-1��
�ʴ�Ϊ��0.25mol•L-1��
��5��A���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ��A��ȷ��
B�����Ʊ���Һʱ���ӿ̶��ߣ���ҺŨ��ƫ����V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫС����B����
C��δ�ñ�Һ��ϴ�ζ��ܣ���Һ��ϡ�ͣ�Ũ�ȼ�С������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ��C��ȷ��
D���ζ�ǰʢ�Ų�����Һ����ƿ������ˮϴ����û�и����V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩��Ӱ�죬��D����
��ѡAC��
���� ���⿼����������ԭ�ζ������ȣ��Ѷ��еȣ�ע��ζ��о������ݹ�ϵʽ���м��㣬���ո��ݹ�ϵʽ���㷽����

 �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�| A�� | ��� | B�� | ǰ�ߴ��ں��� | C�� | ���ߴ���ǰ�� | D�� | ���ж� |
| ʵ����� | 0.1000mol•L1 HCl��Һ���/mL | ����NaOH��Һ���/mL |
| 1 | 27.83 | 20.00 |
| 2 | 25.53 | 20.00 |
| 3 | 27.85 | 20.00 |
�����������������ⶨ���ƫ�ߵ���AC��
A����ʽ�ζ���δ�ñ�������Һ��ϴ
B����ƿδ�ô���Һ��ϴ
C������ζ��յ�ʱ�����Ӷ���
D���ζ�ǰ���ζ����е���ҺҺ����͵��ڡ�0�������£�
| A�� | ���³�ѹ�µ�33.6L������27g����ַ�Ӧ��ת�Ƶ�����ΪС��3NA | |
| B�� | 0.1 mol Cl2��ȫ����ˮ��ת�Ƶĵ�����ĿΪ0.1NA | |
| C�� | 56g��ƬͶ������ŨH2SO4������NA��SO2���� | |
| D�� | 5NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$2HNO3+4N2��+9H2O��Ӧ�У�����28gN2ʱ��ת�Ƶĵ�����ĿΪ3.25NA |
| A�� | X��Y��Z����Ԫ�ؿ��γɻ�����X3YZ4 | |
| B�� | X��Y��Ԫ���γɵĻ�����ֻ��Ϊ���ӻ����� | |
| C�� | �⻯����۷е㣺Y��Z | |
| D�� | �⻯����ȶ��ԣ�Y��Z |
 ��ѧ��Ӧ���ʵĿ����������࣬���¶ȡ�Ũ�ȡ�ѹǿ��������������ȣ����̽���������뻯ѧ��Ӧ���ʹ�ϵ��ʵ����Ƽ��Ľ�Ҳ��������࣮
��ѧ��Ӧ���ʵĿ����������࣬���¶ȡ�Ũ�ȡ�ѹǿ��������������ȣ����̽���������뻯ѧ��Ӧ���ʹ�ϵ��ʵ����Ƽ��Ľ�Ҳ��������࣮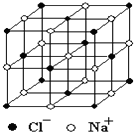 ������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl�ľ�����ͼ��ʾ�����ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ���еIJⶨ���������岽�����£��ٽ�NaCl������ϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У����õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV mL���ش��������⣺
������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl�ľ�����ͼ��ʾ�����ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ���еIJⶨ���������岽�����£��ٽ�NaCl������ϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У����õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV mL���ش��������⣺