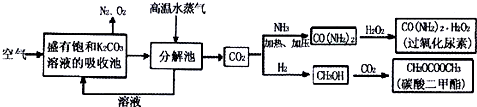
��Ŀ����
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�Na2SO3+S�TNa2S2O3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3?5H2O��40��45���ۻ���48��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ1��ʾ��
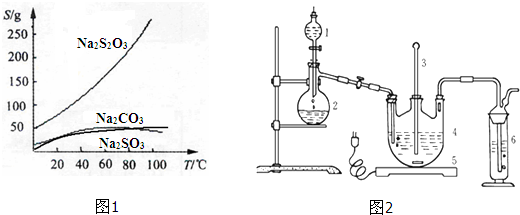
���ְ����·����Ʊ�Na2S2O3?5H2O��
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬����ͼ2��װ��װ�ã�
��1������2������Ϊ ��װ��6�пɷ��� ��
A��BaCl2��Һ B��ŨH2SO4 C������KMnO4��Һ D��NaOH��Һ
��2����Һ©��������ע��Ũ����ʹ��Ӧ�����Ķ�����������Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У����ô������������������ȣ���Ӧԭ��Ϊ��
��Na2CO3+SO2�TNa2SO3+CO2 ��Na2S+SO2+H2O�TNa2SO3+H2S
��2H2S+SO2�T3S��+2H2O ��Na2SO3+S
Na2S2O3
����SO2�����ͨ�룬������Һ���д���dz��ɫ��������������ͨSO2���壬��ӦԼ��Сʱ������Һ��PH�ӽ���С��7ʱ������ֹͣͨ���ͼ��ȣ���ҺPHҪ���Ʋ�С��7�������ǣ� �������ֺ�������ӷ���ʽ��ʾ����
����Na2S2O3?5H2O���ⶨ������

��3��Ϊ���ٲ�Ʒ����ʧ��������Ϊ ���������dz���ϴ�Ӹ������ϴ�Ӳ������� �����Լ�����ϴ�Ӽ���
��4������Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹��� ��
��5���ƵõĴ־��������������������ʣ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3?5H2O�ĺ�����һ�������������������KMnO4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ������ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ʱ������KMnO4��Һ���20.00mL����5S2O32-+8MnO4-+14H+�T8Mn2++10SO42-+7H2O���Իش�
��KMnO4��Һ���� �����ʽ����ʽ�����ζ����У�
�ڵζ��յ�ʱ����ɫ�仯�� ��
�۲�Ʒ��Na2S2O3?5H2O����������Ϊ ��

���ְ����·����Ʊ�Na2S2O3?5H2O��
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬����ͼ2��װ��װ�ã�
��1������2������Ϊ
A��BaCl2��Һ B��ŨH2SO4 C������KMnO4��Һ D��NaOH��Һ
��2����Һ©��������ע��Ũ����ʹ��Ӧ�����Ķ�����������Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У����ô������������������ȣ���Ӧԭ��Ϊ��
��Na2CO3+SO2�TNa2SO3+CO2 ��Na2S+SO2+H2O�TNa2SO3+H2S
��2H2S+SO2�T3S��+2H2O ��Na2SO3+S
| ||
����SO2�����ͨ�룬������Һ���д���dz��ɫ��������������ͨSO2���壬��ӦԼ��Сʱ������Һ��PH�ӽ���С��7ʱ������ֹͣͨ���ͼ��ȣ���ҺPHҪ���Ʋ�С��7�������ǣ�
����Na2S2O3?5H2O���ⶨ������

��3��Ϊ���ٲ�Ʒ����ʧ��������Ϊ
��4������Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹���
��5���ƵõĴ־��������������������ʣ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3?5H2O�ĺ�����һ�������������������KMnO4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ������ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ʱ������KMnO4��Һ���20.00mL����5S2O32-+8MnO4-+14H+�T8Mn2++10SO42-+7H2O���Իش�
��KMnO4��Һ����
�ڵζ��յ�ʱ����ɫ�仯��
�۲�Ʒ��Na2S2O3?5H2O����������Ϊ
���㣺�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������
��������1����������2�Ĺ��켰ʹ�÷���д�������ƣ�����װ��6��ʵ���е�����ѡ���Լ���
��2��������������������������������ӷ�Ӧ���ɶ�����������ʷ�����
��3�������ٵ�Ŀ���Ƿ��������������ɫ���ʵĻ���̿����ò���Ϊ���ˣ����ڳ�������Һ����������Na2S2O3?5H2O��Ϊ�˱����Ʒ��ʧ����Ҫ���ȹ��ˣ�Na2S2O3������ˮ���������Ҵ���Ϊ�˼�����ʧ���������Ҵ�Ϊϴ�Ӽ���
��4�����ݡ�Na2S2O3?5H2O��40��45���ۻ���48��ֽ⡱��������ʱҪ�����¶Ȳ��˹��ߵ�ԭ��
��5�������Ը��������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�ֻ��ʹ����ʽ�ζ��ܣ�
�����ɵĵⵥ���������۱���������������Ƶζ�ʱ������ɫ��ȥ����Ӳ��仯��˵����Ӧ�ﵽ�յ㣻
�۸���n=cV�����1.28g��Ʒ���ĵĸ�����ص����ʵ��������ݸ��ݷ�Ӧ�������Ʒ�к���Na2S2O3?5H2O�����ʵ������ٸ������������ı���ʽ�������Ʒ��Na2S2O3?5H2O������������
��2��������������������������������ӷ�Ӧ���ɶ�����������ʷ�����
��3�������ٵ�Ŀ���Ƿ��������������ɫ���ʵĻ���̿����ò���Ϊ���ˣ����ڳ�������Һ����������Na2S2O3?5H2O��Ϊ�˱����Ʒ��ʧ����Ҫ���ȹ��ˣ�Na2S2O3������ˮ���������Ҵ���Ϊ�˼�����ʧ���������Ҵ�Ϊϴ�Ӽ���
��4�����ݡ�Na2S2O3?5H2O��40��45���ۻ���48��ֽ⡱��������ʱҪ�����¶Ȳ��˹��ߵ�ԭ��
��5�������Ը��������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�ֻ��ʹ����ʽ�ζ��ܣ�
�����ɵĵⵥ���������۱���������������Ƶζ�ʱ������ɫ��ȥ����Ӳ��仯��˵����Ӧ�ﵽ�յ㣻
�۸���n=cV�����1.28g��Ʒ���ĵĸ�����ص����ʵ��������ݸ��ݷ�Ӧ�������Ʒ�к���Na2S2O3?5H2O�����ʵ������ٸ������������ı���ʽ�������Ʒ��Na2S2O3?5H2O������������
���
�⣺��1������ͼʾװ�ÿ�֪������2������Ϊ������ƿ��
װ��6��β������װ����Ҫ���ն���������Ⱦ�����壬ѡ��������KMnO4��Һ���������ԣ��������������������������գ�����������Һ�Ͷ�������Ӧ�����������ƺ�ˮ�������ն�������Ũ���ᡢ�Ȼ������������Ӧ���������ն�����������CD��ȷ��
�ʴ�Ϊ��������ƿ��CD��
��2������ҺpH��7ʱ����Һ��ʾ���ԣ��ᷢ����Ӧ��S2O32-+2H+=S��+SO2+H2O������Na2S2O3�����Ի����в����ȶ����ڣ�Ӧ��ʱ��Һ��pH��С��7��
�ʴ�Ϊ��Na2S2O3�����Ի����в����ȶ����ڣ�S2O32-+2H+=S��+SO2+H2O��
��3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3?5H2O��40��45���ۻ���Ϊ�˱�������Na2S2O3?5H2O���²��ʽ��ͣ����Բ����ٹ��˳�����̿ʱ��Ҫ���ȹ��ˣ�
ϴ�Ӿ���ʱΪ���پ�����ʧ������Na2S2O3?5H2O���ܽ⣬����Na2S2O3������ˮ���������Ҵ�������ѡ���Ҵ�ϴ�ӣ���ϴ�Ӻ��Ҵ��ӷ����������µ����ʣ�
�ʴ�Ϊ�����ȹ��ˣ��Ҵ���
��4������Na2S2O3?5H2O��40��45���ۻ���48��ֽ⣬��������ʱ�¶ȹ��ᵼ�������ľ���ֽ⣬�����˲��ʣ�
�ʴ�Ϊ���¶ȹ��ᵼ�������ľ���ֽ⣻
��5�����������Ը��������Һ�ܹ�������ʽ�ζ��ܵ��ܣ����Եζ����������Ը��������Һ�����ü�ʽ�ζ���ʢ�ţ�Ӧ������ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ��
�����ݱ궨��ԭ����֪�����ɵĵⵥ���������۱���������������Ƶζ�����ɫ��ȥ����Ӳ��仯��˵����Ӧ�ﵽ�յ㣬���Եζ��յ������Ϊ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
�ʴ�Ϊ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
��20mL 0.40mol/L KMnO4��Һ�к��и�����ص����ʵ���Ϊ��n��KMnO4��=0.40mol/L��0.02L=0.008mol��
���ݷ�Ӧ5S2O32-+8MnO4-+14H+�T8Mn2++10SO42-+7H2O��֪��1.28g�Ĵ���Ʒ����Na2S2O3?5H2O�����ʵ���Ϊ��n��Na2S2O3?5H2O��=n��S2O32-��=
��n��KMnO4��=0.005mol��
��Ʒ��Na2S2O3?5H2O����������Ϊ��
��100%=96.9%��
�ʴ�Ϊ��96.9%��
װ��6��β������װ����Ҫ���ն���������Ⱦ�����壬ѡ��������KMnO4��Һ���������ԣ��������������������������գ�����������Һ�Ͷ�������Ӧ�����������ƺ�ˮ�������ն�������Ũ���ᡢ�Ȼ������������Ӧ���������ն�����������CD��ȷ��
�ʴ�Ϊ��������ƿ��CD��
��2������ҺpH��7ʱ����Һ��ʾ���ԣ��ᷢ����Ӧ��S2O32-+2H+=S��+SO2+H2O������Na2S2O3�����Ի����в����ȶ����ڣ�Ӧ��ʱ��Һ��pH��С��7��
�ʴ�Ϊ��Na2S2O3�����Ի����в����ȶ����ڣ�S2O32-+2H+=S��+SO2+H2O��
��3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3?5H2O��40��45���ۻ���Ϊ�˱�������Na2S2O3?5H2O���²��ʽ��ͣ����Բ����ٹ��˳�����̿ʱ��Ҫ���ȹ��ˣ�
ϴ�Ӿ���ʱΪ���پ�����ʧ������Na2S2O3?5H2O���ܽ⣬����Na2S2O3������ˮ���������Ҵ�������ѡ���Ҵ�ϴ�ӣ���ϴ�Ӻ��Ҵ��ӷ����������µ����ʣ�
�ʴ�Ϊ�����ȹ��ˣ��Ҵ���
��4������Na2S2O3?5H2O��40��45���ۻ���48��ֽ⣬��������ʱ�¶ȹ��ᵼ�������ľ���ֽ⣬�����˲��ʣ�
�ʴ�Ϊ���¶ȹ��ᵼ�������ľ���ֽ⣻
��5�����������Ը��������Һ�ܹ�������ʽ�ζ��ܵ��ܣ����Եζ����������Ը��������Һ�����ü�ʽ�ζ���ʢ�ţ�Ӧ������ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ��
�����ݱ궨��ԭ����֪�����ɵĵⵥ���������۱���������������Ƶζ�����ɫ��ȥ����Ӳ��仯��˵����Ӧ�ﵽ�յ㣬���Եζ��յ������Ϊ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
�ʴ�Ϊ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
��20mL 0.40mol/L KMnO4��Һ�к��и�����ص����ʵ���Ϊ��n��KMnO4��=0.40mol/L��0.02L=0.008mol��
���ݷ�Ӧ5S2O32-+8MnO4-+14H+�T8Mn2++10SO42-+7H2O��֪��1.28g�Ĵ���Ʒ����Na2S2O3?5H2O�����ʵ���Ϊ��n��Na2S2O3?5H2O��=n��S2O32-��=
| 5 |
| 8 |
��Ʒ��Na2S2O3?5H2O����������Ϊ��
| 248g/mol��0.005mol |
| 1.28g |
�ʴ�Ϊ��96.9%��
����������ͨ��Na2S2O3?5H2O���Ʊ�����������������ʵ�鷽����Ʒ�����Ϊ�߿��ĸ�Ƶ�⣬�Ѷ��еȣ���ȷ���������Ϣ��ȷ�Ʊ�ԭ��Ϊ��������Ĺؼ��������ֿ�����ѧ���ķ�����������������ѧʵ����������һ�������ϸߵ���Ŀ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��֪X��Y��Z��W��R����Ԫ�أ�ԭ����������������ԭ��������С��20��XԪ�ص�ԭ��������Ԫ�ص�ԭ���а뾶��С�ģ�Y��Wͬ���壬Z��Wͬ���ڣ�YԪ��ԭ�ӵ������������Ǵ�����3����Z��R�ֱ���ͬ�����н�������ǿ��Ԫ�أ�����˵������ȷ���ǣ�������
| A���е㣺X2Y��X2W |
| B����X��Y��Z��W����Ԫ����ɵĻ�����Ⱥ��й��ۼ��ֺ����Ӽ� |
| C��ԭ�Ӱ뾶��X��Y��Z��W��R |
| D��Y��W�γɵĻ�����WY2���γ��������Ҫ����֮һ |
���и���������ָ��������һ���ܴ���������ǣ�������
| A���ں��д���I-���ӵ���Һ�У�Cl-��Fe3+��Na+��Mg2+ |
| B������ˮ�������c��H+��=10-12mol?L-1 ����Һ�У�Na+��Ba2+��Cl-��Br- |
| C��ʹ���ȳʺ�ɫ����Һ�У�Fe2+��Na+��SO42-��ClO- |
| D���ڼ���Al�ܷų�����H2����Һ�У�NH4+��SO42-��Cl-��NO3- |

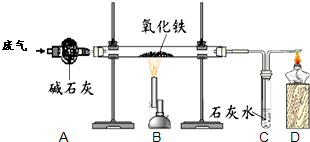 ij����С���ͬѧ�ռ��˺�ˮ������һ����̼�Ͷ�����̼�ķ�����Ϊȷ�����ַ����д���CO��������ʵ���Ұ���ͼ��ʾװ�ý���ʵ�顲����ͨ��װ��A�ٶȺ����������ڴ˴������ķ�Ӧ��ȫ����ʯ�ң�CaO��NaOH�Ļ�����������
ij����С���ͬѧ�ռ��˺�ˮ������һ����̼�Ͷ�����̼�ķ�����Ϊȷ�����ַ����д���CO��������ʵ���Ұ���ͼ��ʾװ�ý���ʵ�顲����ͨ��װ��A�ٶȺ����������ڴ˴������ķ�Ӧ��ȫ����ʯ�ң�CaO��NaOH�Ļ�����������