��Ŀ����
ͭ����Ͻ�����������ʹ�õĽ������ϣ�
��1������ͭ�������� �ѻ���ʽ������ĸ���ţ���
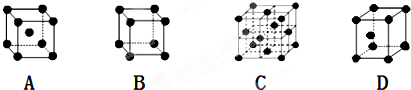
��2����ѧ��ͨ��X�����Ʋ����������������������Ľṹʾ��ͼ�ɼ�ʾ���£�
���������
A����λ�� B�����Ӽ� C�����Թ��ۼ� D�������� E����� F���Ǽ��Թ��ۼ�
��3����ͼ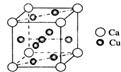 �ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ ����������Ca���������Caԭ���� ����
�ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ ����������Ca���������Caԭ���� ����
��4��Cu2+����NH3��H2O��Cl-���γ���λ��Ϊ4������
�ٳ�ȥAl��OH��3�л��е�����Cu��OH��2��ѡ�������� ��Һ������й��ˡ�ϴ�ӡ����� ��õ�Al��OH��3������Һ�����ɵ������ӵĿռ�ṹͼΪ
������ȥCu��OH��2�л��е�����Al��OH��3�������ѡ���������� ��Һ����Ӧ���������ӷ���ʽΪ ��
��5����ͼ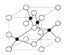 ��ͭ��ij��������ľ����ṹʾ��ͼ���þ�����ܶ�Ϊa g/cm3���谢��٤��������ֵΪNA����þ��������Ϊ cm3��
��ͭ��ij��������ľ����ṹʾ��ͼ���þ�����ܶ�Ϊa g/cm3���谢��٤��������ֵΪNA����þ��������Ϊ cm3��
��1������ͭ��������

��2����ѧ��ͨ��X�����Ʋ����������������������Ľṹʾ��ͼ�ɼ�ʾ���£�

���������
A����λ�� B�����Ӽ� C�����Թ��ۼ� D�������� E����� F���Ǽ��Թ��ۼ�
��3����ͼ
 �ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ
�ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ��4��Cu2+����NH3��H2O��Cl-���γ���λ��Ϊ4������
�ٳ�ȥAl��OH��3�л��е�����Cu��OH��2��ѡ��������
������ȥCu��OH��2�л��е�����Al��OH��3�������ѡ����������
��5����ͼ
 ��ͭ��ij��������ľ����ṹʾ��ͼ���þ�����ܶ�Ϊa g/cm3���谢��٤��������ֵΪNA����þ��������Ϊ
��ͭ��ij��������ľ����ṹʾ��ͼ���þ�����ܶ�Ϊa g/cm3���谢��٤��������ֵΪNA����þ��������Ϊ���㣺�����ļ���,�����ijɼ����,��ͬ����Ľṹ��������������������
ר�⣺��ѧ���뾧��ṹ
��������1��ͭ���������ܶѻ����ݴ��жϣ�
��2�����ݵ�������Ľṹͼ��֪���ھ�����ˮ������ͭ����֮������λ��������֮���Ǽ��Թ��ۼ����������ͭ����֮��Ϊ���Ӽ���ˮ������ˮ����֮����������ݴ˴��⣻
��3�����þ�̯�����㣻���ݾ����ṹ�жϣ�
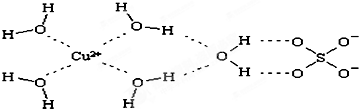
��4����������ͭ���백ˮ�γ�ͭ��������Ӷ�����ˮ��ͭ���ӿ������ĸ��������γ�������ӣ��ݴ˴��⣻
���������������ԣ����Գ�ȥ������������������������Һ������Ԫ�غ͵���غ�д�����ӷ���ʽ��
��5������V=
����������
��2�����ݵ�������Ľṹͼ��֪���ھ�����ˮ������ͭ����֮������λ��������֮���Ǽ��Թ��ۼ����������ͭ����֮��Ϊ���Ӽ���ˮ������ˮ����֮����������ݴ˴��⣻
��3�����þ�̯�����㣻���ݾ����ṹ�жϣ�
��4����������ͭ���백ˮ�γ�ͭ��������Ӷ�����ˮ��ͭ���ӿ������ĸ��������γ�������ӣ��ݴ˴��⣻
���������������ԣ����Գ�ȥ������������������������Һ������Ԫ�غ͵���غ�д�����ӷ���ʽ��
��5������V=
| m |
| �� |
���
�⣺��1��ͭ���������ܶѻ�����ѡC��
��2�����ݵ�������Ľṹͼ��֪���ھ�����ˮ������ͭ����֮������λ��������֮���Ǽ��Թ��ۼ����������ͭ����֮��Ϊ���Ӽ���ˮ������ˮ����֮�����������ѡABCE��
��3�����þ�̯�������֪��������Caλ�ڶ��㣬N��Ca��=8��
=1��Cuλ�����ĺ����ģ�����N��Cu��=8��
+1=5����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ1��5�����ݾ����ṹ��֪����������Ca���������Caԭ�ӷֲ��ھ�������֮���ڵĶ����ϣ�������Caԭ����6�����ʴ�Ϊ��1��5��6��
��4����������ͭ���백ˮ�γ�ͭ��������Ӷ�����ˮ��ͭ���ӿ������ĸ��������γ�������ӣ���ռ�ṹͼΪ��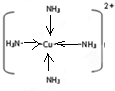 ���ʴ�Ϊ����ˮ��
���ʴ�Ϊ����ˮ�� ��
��
���������������ԣ����Գ�ȥ������������������������Һ������Ԫ�غ͵���غ��д�������������������Ʒ�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ������������Һ��Al��OH��3+OH-=AlO2-+2H2O��
��5�����ݾ�̯����֪����ÿ�������к���ͭԭ����Ϊ8��
+6��
=4����ԭ����Ϊ4������V=
�ɼ�������Ϊ
cm3=
cm3���ʴ�Ϊ
��
��2�����ݵ�������Ľṹͼ��֪���ھ�����ˮ������ͭ����֮������λ��������֮���Ǽ��Թ��ۼ����������ͭ����֮��Ϊ���Ӽ���ˮ������ˮ����֮�����������ѡABCE��
��3�����þ�̯�������֪��������Caλ�ڶ��㣬N��Ca��=8��
| 1 |
| 8 |
| 1 |
| 2 |
��4����������ͭ���백ˮ�γ�ͭ��������Ӷ�����ˮ��ͭ���ӿ������ĸ��������γ�������ӣ���ռ�ṹͼΪ��
 ���ʴ�Ϊ����ˮ��
���ʴ�Ϊ����ˮ�� ��
�����������������ԣ����Գ�ȥ������������������������Һ������Ԫ�غ͵���غ��д�������������������Ʒ�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ������������Һ��Al��OH��3+OH-=AlO2-+2H2O��
��5�����ݾ�̯����֪����ÿ�������к���ͭԭ����Ϊ8��
| 1 |
| 8 |
| 1 |
| 2 |
| m |
| �� |
| ||
| a |
| 320 |
| aNA |
| 320 |
| aNA |
������������Ҫ���龧���Ľṹ�������ṹ�������ļ����֪ʶ����һ�����ۺ��ԣ��е��Ѷȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��һ�����pH=12��Ba��OH��2��Һ�У���μ���һ�����ʵ���Ũ�ȵ�NaHSO4��Һ������Һ�е�Ba2+ǡ����ȫ����ʱ����ҺpH=11������Ӧ����Һ���������Ba��OH��2��Һ��NaHSO4��Һ�����֮�ͣ���NaHSO4��Һ��pH�ǣ���֪lg2=0.3����������
| A��2 | B��2.3 |
| C��2.6 | D��2.9 |
����Һ��pH��4������Һ��pH��5�������Һ������Һ��c��OH-��֮��Ϊ��������
| A��4��5 | B��1��10 |
| C��10��1 | D��1��2 |
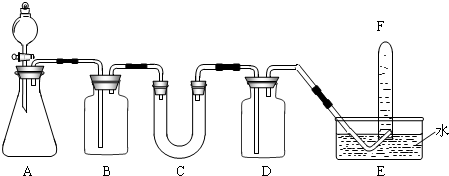
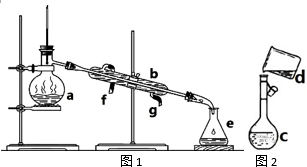 �����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�