��Ŀ����
��������dz��õ�������Ʒ�����ͷ�к�����Ǻ�����أ�����ϸ�۲�ͼ1A��B����ʾ��ʵ��װ�ã����ش����и��⣮

��1�����װ��A��ʵ��Ŀ���� ��ʵ��ʱ����������ȫ���������ձ��������һ��ȼ�ŵĻ���ȼ���ͷ�������ͷ���꣬��������Ƴ���Ѹ�ٽ����ձ����������ձ��У����������ձ����ɹ۲쵽��ʵ�������� ��
��2����ͬѧ��ΪAװ���г��˿�ѡ��ϡ����������ѡ�ã�Ʒ����Һ����ɫʯ����Һ�����з�̪��NaOH��Һ����ˮ�ȣ�����ҷ�������̭����ɫʯ����Һ�͵��з�̪��NaOH��Һ������Ϊ��̭��ԭ������� ����ͬѧ����Ϊ��Aװ����ѡ��Ũ�ĸ������������Һ���ã���ΪŨ��Խ��Ӧ����Խ�죬����Ϊ���� �����ж���˵��ԭ��
��3��װ��B�ɶ����������ͷ����ǵ����������ø�װ�û����Բ�����װ�����м�ȩ�ĺ������䷴Ӧ�����ӷ���ʽΪ��
4MnO4-+12H++5HCHO�T5CO2+4Mn2++11H2O
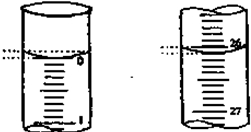
ע����������Ϊ100mL����ͼ1B����ʵ������е�ע������100��ʱ���������Һǡ����ȫ��Ӧ�������װ���ҿ����м�ȩ�ĺ���Ϊ�� mg?m-3��
��4��Ϊ�˲��ؼ�����ܲ�������еļ�ȩ��������ͬѧ�������ͼ2��ʾ��ͼ������������������Һ��ɫʱ�ij���������ÿ��ʵ����ͬ����ע������������ص�����Ϊ4mL������ͬѧ���ø�����ص����ʵ���Ũ��Ϊ�� ��10-5mol?L-1����������λС���� ��ij�βⶨ��װ�����м�ȩ������ʵ��ʱ����ͬѧͨ����¼����������ȷ���þ��ҿ��Ծ�ס�����ҹ涨�����еļ�ȩ�������ó���0.8mg?m-3��������Ϊ��ͬѧ��ʵ���г����Ĵ�������Ϊ �Σ�

��1�����װ��A��ʵ��Ŀ����
��2����ͬѧ��ΪAװ���г��˿�ѡ��ϡ����������ѡ�ã�Ʒ����Һ����ɫʯ����Һ�����з�̪��NaOH��Һ����ˮ�ȣ�����ҷ�������̭����ɫʯ����Һ�͵��з�̪��NaOH��Һ������Ϊ��̭��ԭ�������
��3��װ��B�ɶ����������ͷ����ǵ����������ø�װ�û����Բ�����װ�����м�ȩ�ĺ������䷴Ӧ�����ӷ���ʽΪ��
4MnO4-+12H++5HCHO�T5CO2+4Mn2++11H2O
ע����������Ϊ100mL����ͼ1B����ʵ������е�ע������100��ʱ���������Һǡ����ȫ��Ӧ�������װ���ҿ����м�ȩ�ĺ���Ϊ��
��4��Ϊ�˲��ؼ�����ܲ�������еļ�ȩ��������ͬѧ�������ͼ2��ʾ��ͼ������������������Һ��ɫʱ�ij���������ÿ��ʵ����ͬ����ע������������ص�����Ϊ4mL������ͬѧ���ø�����ص����ʵ���Ũ��Ϊ��
���㣺̽�����ʵ���ɻ�������ʵĺ���,��������Ļ�ѧ����
ר�⣺ʵ����
��������1������������ͷ�к������ȼ�ջ����ɶ����������������ǿ��ԭ�ԣ�ʹ���Ը��������Һ��ɫ���ݴ˷�����
��2�����ݻ��ȼ�տ϶������CO2���������е�CO2��Ҳ��ʹ��ɫʯ����������Ʒ�̪��Һ��ɫ�����Բ����ж��Ƿ��ж����������������Ũ�ĸ������������Һ��Ҫ����Ķ����������ʹ����ɫ������ʵ���ʱ���������ݴ˷�����
��3������ǡ�÷�Ӧ�����꣬����KMnO4��Һ��ɫ��ȥ�����ݻ�ѧ����ʽ4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O���HCHO�����ʵ�����Ȼ��������������������ȩ��Ũ�ȣ�
��4������ͼ��ѡȡ����20�Σ���ȩŨ��Ϊ2.0mg?m-3�����ݻ�ѧ����ʽ4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O���������ص����ʵ���Ũ�ȣ����ݼ�ȩ�������ó���0.8mg?m-3���Ծ�ס��������0.8mg?m-3��ϸ�����ص����ʵ���Ũ�ȼ�������Ĵ�����
��2�����ݻ��ȼ�տ϶������CO2���������е�CO2��Ҳ��ʹ��ɫʯ����������Ʒ�̪��Һ��ɫ�����Բ����ж��Ƿ��ж����������������Ũ�ĸ������������Һ��Ҫ����Ķ����������ʹ����ɫ������ʵ���ʱ���������ݴ˷�����
��3������ǡ�÷�Ӧ�����꣬����KMnO4��Һ��ɫ��ȥ�����ݻ�ѧ����ʽ4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O���HCHO�����ʵ�����Ȼ��������������������ȩ��Ũ�ȣ�
��4������ͼ��ѡȡ����20�Σ���ȩŨ��Ϊ2.0mg?m-3�����ݻ�ѧ����ʽ4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O���������ص����ʵ���Ũ�ȣ����ݼ�ȩ�������ó���0.8mg?m-3���Ծ�ס��������0.8mg?m-3��ϸ�����ص����ʵ���Ũ�ȼ�������Ĵ�����
���
�⣺��1����Ϊ������ͷ�к������ȼ�ջ����ɶ����������������ǿ��ԭ�ԣ�ʹ���Ը��������Һ��ɫ���������װ��A��ʵ��Ŀ������֤���ͷ���Ƿ�����������ͷȼ�պ��Ƿ���SO2���ɣ�������ͷ�к�����ǣ�����ձ��в��������������壬���䵹�������ձ��У����������ձ�����������ԭ���������Һ�����Կɹ۲쵽��ʵ����������ɫ��Ϊ��ɫ��
�ʴ�Ϊ����֤���ͷ���Ƿ�����������ͷȼ�պ��Ƿ���SO2���ɣ���ɫ��Ϊ��ɫ��
��2����Ϊ���ȼ�տ϶������CO2���������е�CO2��Ҳ��ʹ��ɫʯ����������Ʒ�̪��Һ��ɫ�����Բ����ж��Ƿ��ж����������������Ũ�ĸ������������Һ��Ҫ����Ķ����������ʹ����ɫ������ʵ���ʱ��������
�ʴ�Ϊ�����ȼ�ղ�����CO2���������е�CO2��Ҳ��ʹ��ɫʯ����������Ʒ�̪��Һ��ɫ��������ΪŨ��Խ������Ҫ����Ļ��ȼ�գ�������SO2������ʹ���������Һ��ɫ������ʵ���ʱ��������
��3������100�Σ����鵽���������100mL��100=50000mL=10L=0.01m-3�����ݷ�Ӧ���裺��õ������к��м�ȩ�����ʵ�����n����
4MnO4-+5HCHO+12H+�T4Mn2++5CO2+11H2O��
4 5
0.004L��1.00��10-4mol?L-1 n
���n=
=5��10-7mol��������5��10-7mol��30g/mol=1.5��10-5g=0.015mg���þ����ڿ����м�ȩ��Ũ��Ϊ
=1.5mg?m-3��
�ʴ�Ϊ��1.5��
��4������ͼ��ѡȡ����20�Σ���ȩŨ��Ϊ2.0mg?m-3�����Լ�ȩ������Ϊ��2.0mg?m-3��0.1��10-3m3��20=4��10-3mg=4��10-6g�����ݻ�ѧ����ʽ���裺������ص����ʵ���Ũ��ΪC��10-5mol?L-1����
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
4 5
0.004L��C��10-5mol?L-1
��
���C=
=2.67��
���ݼ�ȩ�������ó���0.8mg?m-3���Ծ�ס�������ݼ�ȩ����0.8mg?m-3�����Ĵ������٣����ݻ�ѧ����ʽ���裺�����Ĵ���x�����ȩ������Ϊ��0.8mg?m-3��0.1��10-3m3��x=8x��10-5mg=8x��10-8g��
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
4 5
0.004L��2.67��10-5mol?L-1
��
���x=
=50��
�ʴ�Ϊ��2.67��50��
�ʴ�Ϊ����֤���ͷ���Ƿ�����������ͷȼ�պ��Ƿ���SO2���ɣ���ɫ��Ϊ��ɫ��
��2����Ϊ���ȼ�տ϶������CO2���������е�CO2��Ҳ��ʹ��ɫʯ����������Ʒ�̪��Һ��ɫ�����Բ����ж��Ƿ��ж����������������Ũ�ĸ������������Һ��Ҫ����Ķ����������ʹ����ɫ������ʵ���ʱ��������
�ʴ�Ϊ�����ȼ�ղ�����CO2���������е�CO2��Ҳ��ʹ��ɫʯ����������Ʒ�̪��Һ��ɫ��������ΪŨ��Խ������Ҫ����Ļ��ȼ�գ�������SO2������ʹ���������Һ��ɫ������ʵ���ʱ��������
��3������100�Σ����鵽���������100mL��100=50000mL=10L=0.01m-3�����ݷ�Ӧ���裺��õ������к��м�ȩ�����ʵ�����n����
4MnO4-+5HCHO+12H+�T4Mn2++5CO2+11H2O��
4 5
0.004L��1.00��10-4mol?L-1 n
���n=
| 5��0.004L��1.00��10 -4mol/L |
| 4 |
| 0.015mg |
| 0.01m -3 |
�ʴ�Ϊ��1.5��
��4������ͼ��ѡȡ����20�Σ���ȩŨ��Ϊ2.0mg?m-3�����Լ�ȩ������Ϊ��2.0mg?m-3��0.1��10-3m3��20=4��10-3mg=4��10-6g�����ݻ�ѧ����ʽ���裺������ص����ʵ���Ũ��ΪC��10-5mol?L-1����
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
4 5
0.004L��C��10-5mol?L-1
| 4��10 -6g |
| 30g/mol |
���C=
4��
| ||
| 5��4��10 -8 |
���ݼ�ȩ�������ó���0.8mg?m-3���Ծ�ס�������ݼ�ȩ����0.8mg?m-3�����Ĵ������٣����ݻ�ѧ����ʽ���裺�����Ĵ���x�����ȩ������Ϊ��0.8mg?m-3��0.1��10-3m3��x=8x��10-5mg=8x��10-8g��
4MnO4-+5HCHO+12H+=4Mn2++5CO2��+11H2O
4 5
0.004L��2.67��10-5mol?L-1
| 8x��10 -8g |
| 30g/mol |
���x=
| 5��0.004��2.67��10 -5��30 |
| 4��8��10 -8 |
�ʴ�Ϊ��2.67��50��
������������Ҫ�����˼�ȩ�����ʡ������IJⶨԭ����֪ʶ���Ѷȴ�֪ʶ��϶࣬ע����ݷ���ʽ���м����Լ���λ��ת���DZ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
����֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�ᵼ�²�õ�NaOH��ҺŨ��ƫ�ߵ��ǣ�������
| A���ζ�ǰ�ζ����������ݣ��ζ���������� |
| B����ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ���� |
| C���ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� |
| D����ƿʢװNaOH����Һǰ������ˮϴ�� |
���и���Һ�У��������ʵ���Ũ�ȹ�ϵ������ȷ���ǣ�������
| A����pH=5��������Һϡ��1000������Һ�е�SO42-��H+Ũ�ȵı�ֵԼΪ1��20 |
| B��0.1 mol/L NaHCO3��Һ�У�c��Na+��=c��HCO3-��+c��H2CO3��+2 c��CO32-�� |
| C����0.2 mol/L NaA��Һ��0.1 mol/L������Һ��������������Һ�Լ������У�c��Na+��+c��H+��=c��A-��+c��Cl-�� |
| D��pH=12��Ba��OH��2��Һ��pH=12��Na2CO3��Һ�У���ˮ���������c��H+����� |
 ���㷢�����ڸ��ֹؽ����Լ�����ԭ���������ʹ���ٴ����ƣ�����һ�ֺϳ�·�����£�
���㷢�����ڸ��ֹؽ����Լ�����ԭ���������ʹ���ٴ����ƣ�����һ�ֺϳ�·�����£�
 ���ĺϳ�·�ߣ�
���ĺϳ�·�ߣ�