��Ŀ����
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ���������Һ�ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ�������һ���������Һ�� ��Ϊ ���Ұ�����ڲ���ɫ��������ζ��յ㣮
��2�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���
A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
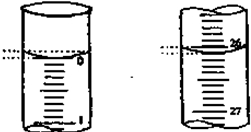
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������������Һ�����Ϊ mL��
��4��ijѧ����������ʵ��ֱ��¼�й��������±�����ѡ�����к�����������ʽ���������������Һ�����ʵ���Ũ�ȣ�����4λС������C��NaOH��= ��
��5����25��ʱ����pHΪx�������pHΪy��NaOH��Һ��ȡV1L���������NaOH��Һ�кͣ���V2L NaOH��Һ���Իش𣺣�����x��6��y��8����
����x+y=12ʱ����V1/V2=
����x+y��14ʱ����V1/V2= �������ʽ������V1 V2�����������������=����
��1���ñ���������Һ�ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��
��2�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���
A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������������Һ�����Ϊ

��4��ijѧ����������ʵ��ֱ��¼�й��������±�����ѡ�����к�����������ʽ���������������Һ�����ʵ���Ũ�ȣ�����4λС������C��NaOH��=
| ����� | ������������ �����/ml | 0.1000mol?L-1��������/ml | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/ml | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 34.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
����x+y=12ʱ����V1/V2=
����x+y��14ʱ����V1/V2=
���㣺�к͵ζ�
ר�⣺ʵ����
��������1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��2������c�����⣩=
����������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��3�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��4���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ��������NaOH��Ӧ���C��NaOH����
��5����������к�ʱH+�����ʵ�����OH-���ӵ����ʵ�����������
��2������c�����⣩=
| V(��)��c(��) |
| V(����) |
��3�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��4���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ��������NaOH��Ӧ���C��NaOH����
��5����������к�ʱH+�����ʵ�����OH-���ӵ����ʵ�����������
���
�⣺��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ƿ����Һ��ɫ�ı仯����ɫ����ɫ��
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��ϡ�ͣ�Ũ�ȼ�С�����V������ƫ����c�����⣩=
��֪��c�����⣩ƫ��A����
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
��֪���ⶨc��NaOH����Ӱ�죬��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=
��֪��c�����⣩ƫ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=
��֪��c�����⣩ƫС����D��ȷ��
��ѡD��
��3����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL���ʴ�Ϊ��26.10��
��4���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=26.10mL��
HCl+NaOH=NaCl+H2O
0.0261L��0.1000mol?L-1 0.025L��C��NaOH��
��C��NaOH��=0.1044mol?L-1��
�ʴ�Ϊ��0.1044mol?L-1��
��5����Ϊ�����pH=x������c��H+��=10-x mol?L-1��pH=y��NaOH��pOH=14-y������c��OH-��=10-��14-y��=10y-14������к�ʱH+�����ʵ�����OH-���ӵ����ʵ�����ȣ�����10-x��Vx=10y-14��Vy����
=
=10x+y-14��
����x+y=12ʱ����
=
=10x+y-14=
��
����x+y��14ʱ����
=
=10x+y-14��100=1������V1��V2��
�ʴ�Ϊ��
��10x+y-14������
�ʴ�Ϊ����ƿ����Һ��ɫ�ı仯����ɫ����ɫ��
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��ϡ�ͣ�Ũ�ȼ�С�����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
| V(��)��c(��) |
| V(����) |
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=
| V(��)��c(��) |
| V(����) |
��ѡD��
��3����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL���ʴ�Ϊ��26.10��
��4���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=26.10mL��
HCl+NaOH=NaCl+H2O
0.0261L��0.1000mol?L-1 0.025L��C��NaOH��
��C��NaOH��=0.1044mol?L-1��
�ʴ�Ϊ��0.1044mol?L-1��
��5����Ϊ�����pH=x������c��H+��=10-x mol?L-1��pH=y��NaOH��pOH=14-y������c��OH-��=10-��14-y��=10y-14������к�ʱH+�����ʵ�����OH-���ӵ����ʵ�����ȣ�����10-x��Vx=10y-14��Vy����
| VX |
| Vy |
| 10y-14 |
| 10-x |
����x+y=12ʱ����
| VX |
| Vy |
| 10y-14 |
| 10-x |
| 1 |
| 10 |
����x+y��14ʱ����
| VX |
| Vy |
| 10y-14 |
| 10-x |
�ʴ�Ϊ��
| 1 |
| 10 |
������������Ҫ�������к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ��������к�ʱH+�����ʵ�����OH-���ӵ����ʵ�������ǽ���ؼ���

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
���и���������ָ��������һ���ܴ���������ǣ�������
| A����̼��������Һ�У�K+��Al3+��SO42-��Cl- |
| B�����ڽ϶�Fe3+����Һ�У�Na+��SCN-��CO32-��K+ |
| C����������ˮ�������C��H+��ˮ?C��OH-��ˮ=10-24��Һ�У�NH4+��Cl-��CO32-��F- |
| D������ʹpH��ֽ������ɫ����Һ�У�Na+��S2-��CO32-��NO3- |
��ij�㶨�¶��£����ݻ�Ϊ1L��������Ͷ��1mol CO��2mol H2O���������·�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����ƽ��ʱ����CO2
mol���������¶Ⱥ��ݻ����䣬����������2mol H2O��g����ʹ��Ӧ�����µ�ƽ�⣬����˵������ȷ���ǣ�������
| 2 |
| 3 |
| A���¡���ƽ��ʱ�����������ѹǿ֮����5��3 |
| B����ƽ��ʱH2O��ת����Ϊ20% |
| C����ƽ��ʱCO��Ũ����0.2 mol?L-1 |
| D���¡���ƽ��ʱ�����������ܶ�֮��Ϊ5��3 |
��ij��������ȡҺ�����ʱ��ƽ��ʱ����ΪnmL������ʱ����Ϊx mL������ʱ����Ϊy mL����X��n��y�������õ���������Ϊ��������
| A����Ͳ | B������ƿ |
| C���ζ��� | D�����Ͼ����� |
�ܹ�������������ˮ�⣬������Է���������ͬ���������ʵ��ǣ�������
| A������ | B������ |
| C������ | D����ѿ�� |
