��Ŀ����
9������ṹ��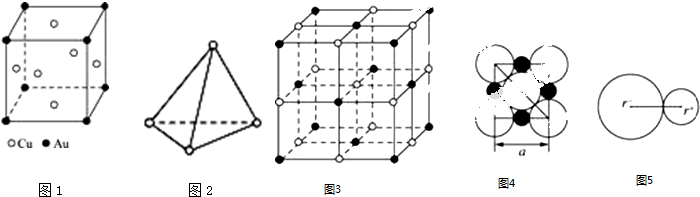
��1�����ͭ�����γɶ��ֽ������������һ�ֵľ���ṹ��ͼ1��ʾ��Ϊ���������ṹ�����ý���������Ļ�ѧʽCu3Au��
��2�����ף�P4������Ϊ���������ͷ��ӣ���ͼ2��ʾ��4��Pԭ��λ������������ĸ����㣬��ش��������⣺
��1mol P4��������6mol P-P��
��P4�����ǷǼ��ԣ���Ի�Ǽ��ԣ�����
��3�������Ӿ����У����������Ӱ�һ�������ڿռ����У�ͼ3��NaCl�ľ���ṹ�������Ӿ����У��������Ӿ��л�ӽ�������ԳƵĵ����ƣ����ǿ��Ա������Dz��Ⱦ��ĸ���Բ���˴����У�ͼ4�������Ӽ��ļ������������������ӵİ뾶֮�ͣ���ͼ5������֪aΪ������
�Իش��������⣺
����NaCl�����У�ÿ��Na+ͬʱ������6Cl-����Na+��Ŀ��Cl-��Ŀ֮��Ϊ1��1��
��Na+�뾶��Cl-�뾶֮��$\frac{{r}^{+}}{{r}^{-}}$=0.414������֪$\sqrt{2}$=1.414��$\sqrt{3}$=1.732��$\sqrt{5}$=2.236��
���� ��1�����þ�̯�����㻯ѧʽ��
��2��P4����Ϊ���������ͷ��ӣ�P4��������6��P-P����P4����Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
��3�������þ�̯������ÿ�������к��е������Ӻ������Ӹ�����
�ڸ���ͼƬ��ȷ��NaCl�������������ӵ���̾��룮
��� �⣺��1��Auԭ��λ�ڶ��㣬��ĿΪ8��$\frac{1}{8}$=1��Cuλ�ھ������ģ���ĿΪ6��$\frac{1}{2}$=3����ѧʽ��дΪ��Cu3Au��
�ʴ�Ϊ��Cu3Au��
��2����P4����Ϊ���������ͷ��ӣ�ÿ����Ϊ�������Σ�P4��������6��P-P����1mol P4��������6mol P-P�����ʴ�Ϊ��6��
��P4����Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
�ʴ�Ϊ���Ǽ��ԣ�
��3���ٸ��ݾ���ͼ����NaCl�����У�ÿ��Na+ͬʱ����6�������ӣ�ÿ���þ����������Ӹ���=12��$\frac{1}{4}$=4�������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����Ӹ�����Ϊ1��1��
�ʴ�Ϊ��6��1��1��
�ڸ���ͼƬ��֪��NaCl�������������ӵ���̾���Ϊa��һ�뼴$\frac{a}{2}$�������ӵİ뾶Ϊ�Խ��ߵ�$\frac{1}{4}$����Ϊ$\frac{\sqrt{2}a}{4}$�������ӵİ뾶Ϊ$\frac{a}{2}-\frac{\sqrt{2}a}{4}$������Na+���Ӱ뾶��Cl-���Ӱ뾶֮��Ϊ $\frac{{r}^{+}}{{r}^{-}}$=$\frac{\frac{a}{2}-\frac{\sqrt{2}a}{4}}{\frac{\sqrt{2}a}{4}}$=0.414��
�ʴ�Ϊ��0.414��
���� ���⿼���йؾ���ļ��㣬��������н�ǿ�ķ����Ժ����ԣ�ѧϰ��ע�⾧�����ȷ���������ѧ���жϾ�������λ���ļ��㣮

��� | ʱ��/min ���ʵ���/mol �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 |
| 1 | 800 | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 |
| 2 | 800 | 1.0 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 |
| 3 | 820 | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 |
��2��ʵ��1�У�0〜l0min������H2��ƽ����Ӧ����Ϊ0.005 mol•L-1•min-1��
��3��ʵ��3�ķ�Ӧ�ﵽ��ѧ��Ӧ��ʱ��HI��g��ת����Ϊ80%��
| A�� | ��Ϊp����ǡ�8�����εģ�����p�ĵ����ߡ�8������ | |
| B�� | K�ܼ���3s��3p��3d��3f�ĸ���� | |
| C�� | ̼ԭ�ӵ�2p����������������෴�ĵ��� | |
| D�� | ����˵��������ȷ |
| A | ||||
| E | B | C | D |
��2��A����Ԫ�����γ�ԭ�����ʵ���֮��Ϊ1��1�Ļ�����仯ѧʽΪH2O2��
��3��C������������ˮ������E���ʵķ�Ӧ���ڷ��ȷ�Ӧ������ȡ����ȡ������������������С�ڣ�����ڡ�����С�ڡ����ڡ�����Ӧ�����������
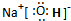 �������еĻ�ѧ�������Ӽ������Թ��ۼ������������ӻ��������ӡ����ۡ�����
�������еĻ�ѧ�������Ӽ������Թ��ۼ������������ӻ��������ӡ����ۡ�����