��Ŀ����
13�������й�˵����ȷ���ǣ�������| A�� | �����£�0.1 mol•L-1Na2S��Һ�д��ڣ�c��OH-��=c��H+��+c��HS-��+c��H2S�� | |
| B�� | pH=3�Ĵ�����Һ�У�c��H+��=c��CH3COO-��=3.0mol•L-1 | |
| C�� | ij��Һ����ˮ�������c��OH-��=1��10-amol•L-1����a��7ʱ�������Һ��pH����Ϊa | |
| D�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-����c��NH4+ ����c��H+����c��OH-�� |
���� A���κε������Һ�ж����������غ㣬���������غ��жϣ�
B���κε������Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
C����������ˮ���룻
D�������£�pH=12�İ�ˮ��ҺŨ�ȴ���pH=2�����ᣬ�������Ϻ�ˮ��ʣ�࣬��Һ�ʼ��ԣ�
��� �⣺A���κε������Һ�ж����������غ㣬���������غ�֪��Na2S��Һ�д��ڣ�c��OH-��=c��H+��+c��HS-��+2 c��H2S������A����
B��pH=3�Ĵ�����Һ�У�c��H+��=10-3mol/L�����ݵ���غ��c��H+��=c��OH-��+c��CH3COO-��������c��H+����c��CH3COO-������B����
C��a��7ʱ��ˮ�ĵ��뱻���ƣ�����Ϊ�����ҺpHֵ������a��14-a����C��ȷ��
D��pH=2��������pH=12�İ�ˮ��������ʱ����ˮ������������Һ�У�c��NH4+ ����c��Cl-����c��OH-����c��H+������D����
��ѡC��
���� ���⿼������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬��ȷ��Һ�����ʳɷּ��������ǽⱾ��ؼ���������Ũ�ȴ�С�Ƚ��г���Ӧ�õ���غ�������غ㣬֪����Щ��֮����������غ㣬���ؿ�������ж��������״�ѡ����C��

��ϰ��ϵ�д�
�����Ŀ
4�������ķ�����ȥ��2����ԭ���γ�һ��˫�������ȷ�Ӧ����Լ��Ҫ117kJ/mol��125kJ/mol����������1��3-������ϩʧȥ2����ԭ�ӱ�ɱ��Ƿ��ȷ�Ӧ����Ӧ��Ϊ23.4kJ/mol��������ʵ������������
| A�� | 1��3-������ϩ���������ȷ�Ӧ | |
| B�� | ��ȫȼ��ʱ1��3-������ϩ�ȱ����ȶ� | |
| C�� | ���������ɻ����������ȷ�Ӧ | |
| D�� | ����1��3-������ϩ�ȶ� |
1��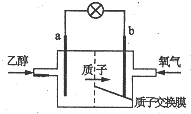 �����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������
�����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������
 �����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������
�����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������| A�� | ��ع���ʱ������b���ص��߾����ݵ�a�� | |
| B�� | a��Ϊ��صĸ������õ缫����������Ӧ | |
| C�� | ��������ĵ缫��ӦʽΪO2+2H2O+4e-=4OH- | |
| D�� | ��ع���ʱ��23g�Ҵ�������ת�Ƶ���ʽΪ6mol |
18����ʵ���ҽ��н̲����ص���ʾʵ�飬�����ڵ�ʵ���������Լ������õ����ǣ�������
| A�� | ���ǵ�ˮ�⣨�Թܡ�������Һ�����Ƶ�Cu��OH��2����Һ�� | |
| B�� | ��NaOH����Һȷ��δ֪Ũ�ȵ�������Һ��ʯ����Һ����ʽ�ζ��ܡ���ƿ�� | |
| C�� | ֤�������д��ڵ�Ԫ�أ�©����ϡ���ᡢ�������������� | |
| D�� | �������������壨����FeCl3��Һ��NaOH��Һ����ͷ�ιܣ� |
5��ij����ѧϰС���ͬѧ��������������ԭ��Ӧ���ԭ��أ�2KMnO4+10FeSO4+8H2SO4�T2MnSO4+5Fe2��SO4��3+K2SO4+8H2O������װ�б���K2SO4��Һ��������������ȷ���ǣ�������


| A�� | b�缫�Ϸ�����ԭ��Ӧ | |
| B�� | ���ձ�����Һ��pH��С | |
| C�� | ��ع���ʱ�������е�SO42-������ձ� | |
| D�� | ���·�ĵ��������Ǵ�a��b |
2����֪�Ȼ�����Һ��⻯����Һ���ʱ������Ӧ��2Fe3+��aq��+2I-��aq��=2Fe2+��aq��+I2��s�����ֽ�1 L 0.25mol•L-1�Ȼ�����Һ��4 L 0.10 mol•L-�⻯����Һ��Ϻ��ڵ�2minʱ����û����Һ��c��I-��=0.04 mo1•L-��������˵����ȷ���ǣ�������
| A�� | ��2minʱ��c��Fe2+��=0.01 mol•L-1 | |
| B�� | �����Һ�У�c��K+��=0.01 mol•L-1 | |
| C�� | 0��2min�ڣ�v��I-��=0.01 mol•L-1•min-1 | |
| D�� | ��2minʱ��c��Fe3+��=0.01 mol•L-1 |
6������ʱ����Ũ�Ⱥ�����ֱ�ΪC1��V1��NaOH��Һ��C2��V2��CH3COOH��Һ���ϣ����й��ڸû����Һ������������ǣ�������
| A�� | ��pH��7ʱ����һ����C1V1��C2V2 | |
| B�� | ��pH��7ʱ�������Һ�п�����c��Na+����c��H+�� | |
| C�� | ��pH=7ʱ����V1=V2����һ����C2=C1 | |
| D�� | �� V1=V2��C1=C2����c��CH3COO-��+c��CH3COOH��=c��Na+�� |