��Ŀ����
4��������Ԫ��W��X��Y��Z��ԭ������������������W�������Ӻ��������X��Y��Zԭ�ӵĺ����ڲ��������ͬ��X��һ�ֺ����ڿ���ʱ��������������������Yԭ�ӵ��������������ڲ���ӵ�3������Z�����γ�˫ԭ�ӷ��ӣ�����˵����ȷ���ǣ�������| A�� | W��X��Y��Zԭ�ӵĺ����������������ܺ�Ϊ19 | |
| B�� | W�������Ӱ뾶С��Li+ | |
| C�� | W��Y���γɼȺ����Ӽ��ֺ����ۼ��Ļ����� | |
| D�� | X��Y�ļ���̬�⻯����ȶ��ԣ�X��Y |
���� ������Ԫ��W��X��Y��Z��ԭ��������������X��һ�ֺ����ڿ���ʱ����������������������X��CԪ�أ�
Yԭ�ӵ��������������ڲ���ӵ�3����ΪOԪ�أ�W�������Ӻ��������X��Y��Zԭ�ӵĺ����ڲ��������ͬ����WΪHԪ�أ�
��Z�����γ�˫ԭ�ӷ��ӣ���ZΪNe��ArԪ�أ�
A��W��X��Y��Zԭ�������������ֱ���1��4��6��8��
B�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С��
C��W��HԪ�ء�Y��OԪ�أ��ǽ���Ԫ��֮�����γɹ��ۼ���
D��X��C��Y��OԪ�أ�Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ��
��� �⣺������Ԫ��W��X��Y��Z��ԭ��������������X��һ�ֺ����ڿ���ʱ����������������������X��CԪ�أ�
Yԭ�ӵ��������������ڲ���ӵ�3����ΪOԪ�أ�W�������Ӻ��������X��Y��Zԭ�ӵĺ����ڲ��������ͬ����WΪHԪ�أ�
��Z�����γ�˫ԭ�ӷ��ӣ���ZΪNe��ArԪ�أ�
A��W��X��Y��Zԭ�������������ֱ���1��4��6��8������������Ԫ��ԭ������������=1+4+6+8=19����A��ȷ��
B�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С���������ӱȽ�H-��Li+����B����
C���ǽ���Ԫ��֮�����γɹ��ۼ���W��HԪ�ء�Y��OԪ�أ����Զ������γɹ��ۻ������C����
D��X��C��Y��OԪ�أ�Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ���ǽ�����Y��X������X��Y�ļ���̬�⻯����ȶ��ԣ�Y��X����D����
��ѡA��
���� ���⿼��ԭ�ӽṹ��Ԫ�������ɣ�Ϊ��Ƶ���㣬�漰Ԫ���ƶϡ���ѧ�����ǽ�����ǿ���Ƚϡ����Ӱ뾶��С�Ƚϵ�֪ʶ�㣬��ȷԭ�ӽṹ��Ԫ�������ɼ����ʹ�������֪ʶ���ǽⱾ��ؼ����״�ѡ����B����Ŀ�ѶȲ���

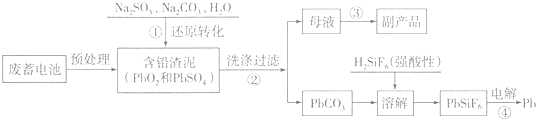
��֪��Ksp��PbSO4��=1.6��10-5��Ksp��PbCO3��=3.3��10-14��
�ش��������⣺
��1��д���������PbSO4ת��ΪPbCO3���̵�ƽ�ⳣ������ʽK=$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$��Ϊ��߲���ٵķ�Ӧ���ʺ�Ǧ�����ʣ�����Ϊ�ɲ�ȡ��������ʩ�dz�ֽ��衢�ʵ������¶ȣ�
��2��������з�����������ԭ��Ӧ�����ӷ���ʽΪPbO2+SO32-+H2O=PbSO4+2OH-��
��3��д��������ö��Ե缫����������ӦʽPb2++2e-=Pb��
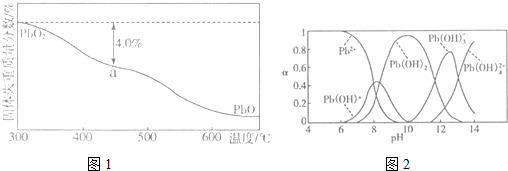
��4��PbO2�ڼ��ȹ��̷����ֽ��ʧ��������ͼ1��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0%����$\frac{��Ʒ��ʼ����-a���������}{��Ʒ��ʼ����}$��100%���IJ������壬��a�������ɱ�ʾΪPbOx������x=1.4��
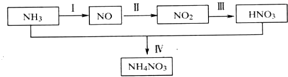
��5��Ǧ�ļӹ�ͬ����ʹˮ�����ؽ���Ǧ�ĺ����������������Ⱦ��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2��Pb��OH��3-��Pb��OH��42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ��ͼ2��ʾ��ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������
| ����/��mol��L-1�� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.9 |
A��4��5 B��6��7 C��9��10 D��11��12��


 2NH3��+CO2��+H2O
2NH3��+CO2��+H2O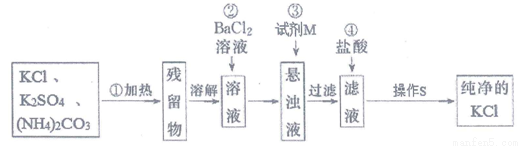

 ����ӵ�ع㷺Ӧ�����ճ����Ӳ�Ʒ�У�Ҳ�ǵ綯����������ص���ѡ���������ϵ�ѡ�����������ӵ�ص����ܣ��������أ�LiFePO4������߱����ԡ��߱���������ѭ�����ԡ��߰�ȫ�ԡ��ͳɱ����������ŵ����Ϊ����Դ���ǡ���
����ӵ�ع㷺Ӧ�����ճ����Ӳ�Ʒ�У�Ҳ�ǵ綯����������ص���ѡ���������ϵ�ѡ�����������ӵ�ص����ܣ��������أ�LiFePO4������߱����ԡ��߱���������ѭ�����ԡ��߰�ȫ�ԡ��ͳɱ����������ŵ����Ϊ����Դ���ǡ���

 ��
�� ��
��