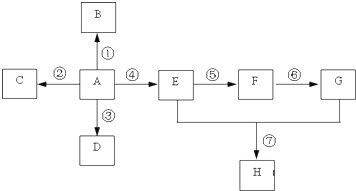
��Ŀ����
 ��֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
��֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ����1��A��һ�������¿��Ծۺ�����һ�ֳ������ϣ������ϵĽṹ��ʽΪ
��2����Ӧ�ܵĻ�ѧ����ʽΪ
��3����Ҫ��ش�
�١��ܵķ�Ӧ����Ϊ
��4����ʵ�����л�õ�����������������B��D��Ϊ�ᴿE��������Լ��Լ��������������
��5������٢ڢ۲�ת���ʶ���100%���ڢܲ�ת������60%�����ñ�״����4.48��105m3��A���������������������������������������
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
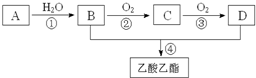
������A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ϩ��ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH��B�������õ�CΪCH3CHO��C�����õ�DΪCH3COOH���Ҵ������ᷢ��������Ӧ�õ������������ݴ˽��
���
�⣺A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH��B�������õ�CΪCH3CHO��C�����õ�DΪCH3COOH���Ҵ������ᷢ��������Ӧ�õ�����������
��1��AΪ��ϩ������̼̼˫���������Ӿ۷�Ӧ�õ�����ϩ���ṹ��ʽΪ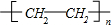 ���ʴ�Ϊ��
���ʴ�Ϊ��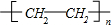 ��
��
��2����Ӧ�ܵĻ�ѧ����ʽΪ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��3����Ӧ������ϩ���Ƿ����ӳɷ�Ӧ�����Ҵ�����Ӧ��Ԫ�����Ҵ�����������Ӧ��������������Ҳ����ȡ����Ӧ����Ӧ�����Ҵ��ڴ��������������·�������������ȩ���ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��ȡ����Ӧ�����������ȣ�
��4����ʵ�����л�õ������������������Ҵ������ᣬΪ�ᴿ�����������ñ���̼������Һ�������ᡢ�Ҵ�������������ˮ��Һ�����ܣ����÷�Һ�ķ������з��룬�ʴ�Ϊ������̼������Һ����Һ��
��5������٢ڢ۲�ת���ʶ���100%���ڢܲ�ת������60%����״����4.48��105m3����ϩΪ
=2��107mol����CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O����֪�������Ҵ������ʵ���֮��Ϊ1��1����������������Ϊ2��107mol��
CH3COOCH2CH3+H2O����֪�������Ҵ������ʵ���֮��Ϊ1��1����������������Ϊ2��107mol��
��60%��88g/mol=5.28��108g=528�֣��ʴ�Ϊ��528��
��1��AΪ��ϩ������̼̼˫���������Ӿ۷�Ӧ�õ�����ϩ���ṹ��ʽΪ
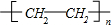 ���ʴ�Ϊ��
���ʴ�Ϊ��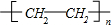 ��
����2����Ӧ�ܵĻ�ѧ����ʽΪ��CH3COOH+CH3CH2OH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����3����Ӧ������ϩ���Ƿ����ӳɷ�Ӧ�����Ҵ�����Ӧ��Ԫ�����Ҵ�����������Ӧ��������������Ҳ����ȡ����Ӧ����Ӧ�����Ҵ��ڴ��������������·�������������ȩ���ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��ȡ����Ӧ�����������ȣ�
��4����ʵ�����л�õ������������������Ҵ������ᣬΪ�ᴿ�����������ñ���̼������Һ�������ᡢ�Ҵ�������������ˮ��Һ�����ܣ����÷�Һ�ķ������з��룬�ʴ�Ϊ������̼������Һ����Һ��
��5������٢ڢ۲�ת���ʶ���100%���ڢܲ�ת������60%����״����4.48��105m3����ϩΪ
| 4.48��108L |
| 22.4L/mol |
 CH3COOCH2CH3+H2O����֪�������Ҵ������ʵ���֮��Ϊ1��1����������������Ϊ2��107mol��
CH3COOCH2CH3+H2O����֪�������Ҵ������ʵ���֮��Ϊ1��1����������������Ϊ2��107mol��| 1 |
| 2 |
���������⿼�������ƶϡ������������Ʊ�����ѧ����ʽ�йؼ���ȣ��漰ϩ��������ȩ�������������ת�����Ƚϻ��������ضԻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A��Ħ�������ʵ�������λ |
| B��������Ħ��������2g |
| C��1molNH3��������17g |
| D��1mol������ռ�����ԼΪ22.4L |