��Ŀ����
����A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ����������������֪A��E��D��G�ֱ�ͬ���壻E��F��G��Hͬ���ڣ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N��B������������������Ӳ�����2����D�ǵؿ��к�������Ԫ�أ�Fλ��B��ǰһ���壮��ش��������⣺
��1��Ԫ��B�����ڱ��е�λ�� ��M�Ŀռ乹���� ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ ����ҵ������ijһ����Ӧ��ͬʱ�����û������H�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ ��
��4��M��N���ܽ��H+�����н��H+������ǿ���� ���ѧʽ����N���H+���γɵ�������ԭ�Ӳ��� �ӻ�������DZ�N�еļ��Ǵ�ԭ��Ϊ ��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ���� �������ӷ���ʽ��ʾ����������7.8g X��ˮ��Ӧ�ų�Q kJ������Q��0����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1��Ԫ��B�����ڱ��е�λ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ
��4��M��N���ܽ��H+�����н��H+������ǿ����
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ����
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ��������������D�ǵؿ��к�������Ԫ�أ�����D����Ԫ�أ�D��Gͬ���壬����G����Ԫ�أ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ��������AΪ��Ԫ�أ�CΪ��Ԫ�أ�A��E���壬E��F��G��Hͬ���ڣ�����EΪ��Ԫ�أ�HΪ��Ԫ�أ�B������������������Ӳ�����2��������BΪ̼Ԫ�أ�Fλ��B��ǰһ���壬����FΪ��Ԫ�أ��ݴ˴��⣮
���
�⣺A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ��������������D�ǵؿ��к�������Ԫ�أ�����D����Ԫ�أ�D��Gͬ���壬����G����Ԫ�أ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ��������AΪ��Ԫ�أ�CΪ��Ԫ�أ�A��E���壬E��F��G��Hͬ���ڣ�����EΪ��Ԫ�أ�HΪ��Ԫ�أ�B������������������Ӳ�����2��������BΪ̼Ԫ�أ�Fλ��B��ǰһ���壬����FΪ��Ԫ�أ�
��1��Ԫ��BΪ̼Ԫ�أ�λ�����ڱ��е�λ�õ�2���ڢ�A�壬MΪ���������ڵ�ԭ�ӵļ۲���Ӷ���Ϊ
=4����һ�Թµ��Ӷԣ��������Ŀռ乹���� �����Σ�
�ʴ�Ϊ����2���ڢ�A�壻 �����Σ�
��2��A��D��E����Ԫ�����һ�ֳ���������ΪNaOH����WΪ�ǻ������ĵ���ʽΪ ����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH���������÷�Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O
����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH���������÷�Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O
2NaOH+Cl2��+H2�����ʴ�Ϊ�� ��2NaCl+2H2O
��2NaCl+2H2O
2NaOH+Cl2��+H2����
��3��NaOH��Al��OH��3֮�䷴Ӧ�����ӷ���ʽΪ Al��OH��3+OH-�TAlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-�TAlO2-+2H2O��
��4��M��N�ֱ�Ϊ������ˮ�����ܽ��H+����Ϊ��ԭ�ӵ縺�Աȵ�ԭ�ӵ縺�Դµ��ӶԸ�����ԭ�Ӻˣ���ԭ����λ�����ϵ�������˽��H+������ǿ���� NH3��H3O+������ԭ��O����sp3�ӻ����γ�4���ӻ������H2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С����H3O+���DZ�H2O�еļ��Ǵ�
�ʴ�Ϊ��NH3��sp3��H2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С��
��5��X��Y�ֱ�ΪNa2O2��Na2S������Na2S��ˮ��Һ����������ˮ���ʹ��Һ�Լ��ԣ������ӷ���ʽΪS2-+H2O?HS-+OH-��������7.8g Na2O2����Ϊ0.1mol������ˮ��Ӧ�ų�Q kJ������Q��0������2molNa2O2��ˮ��Ӧ�ų�20Q kJ���ȣ����Ը÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2��s��+2H2O��1���T4NaOH��aq��+O2��g����H=-20Q kJ/mol���ʴ�Ϊ��S2-+H2O?HS-+OH-��2Na2O2��s��+2H2O��1���T4NaOH��aq��+O2��g����H=-20Q kJ/mol��
��1��Ԫ��BΪ̼Ԫ�أ�λ�����ڱ��е�λ�õ�2���ڢ�A�壬MΪ���������ڵ�ԭ�ӵļ۲���Ӷ���Ϊ
| 5+3 |
| 2 |
�ʴ�Ϊ����2���ڢ�A�壻 �����Σ�
��2��A��D��E����Ԫ�����һ�ֳ���������ΪNaOH����WΪ�ǻ������ĵ���ʽΪ
 ����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH���������÷�Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O
����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH���������÷�Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O
| ||
 ��2NaCl+2H2O
��2NaCl+2H2O
| ||
��3��NaOH��Al��OH��3֮�䷴Ӧ�����ӷ���ʽΪ Al��OH��3+OH-�TAlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-�TAlO2-+2H2O��
��4��M��N�ֱ�Ϊ������ˮ�����ܽ��H+����Ϊ��ԭ�ӵ縺�Աȵ�ԭ�ӵ縺�Դµ��ӶԸ�����ԭ�Ӻˣ���ԭ����λ�����ϵ�������˽��H+������ǿ���� NH3��H3O+������ԭ��O����sp3�ӻ����γ�4���ӻ������H2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С����H3O+���DZ�H2O�еļ��Ǵ�
�ʴ�Ϊ��NH3��sp3��H2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С��
��5��X��Y�ֱ�ΪNa2O2��Na2S������Na2S��ˮ��Һ����������ˮ���ʹ��Һ�Լ��ԣ������ӷ���ʽΪS2-+H2O?HS-+OH-��������7.8g Na2O2����Ϊ0.1mol������ˮ��Ӧ�ų�Q kJ������Q��0������2molNa2O2��ˮ��Ӧ�ų�20Q kJ���ȣ����Ը÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2��s��+2H2O��1���T4NaOH��aq��+O2��g����H=-20Q kJ/mol���ʴ�Ϊ��S2-+H2O?HS-+OH-��2Na2O2��s��+2H2O��1���T4NaOH��aq��+O2��g����H=-20Q kJ/mol��
������������Ҫ�����˲��ֽ����ͷǽ�����Ԫ�ػ�����֪ʶ���ѶȲ�����Ĺؼ����ڸ�����Ŀ�е���Ϣȷ��Ԫ�ص����࣬�Լ�����ʱע�⻯ѧ����Ĺ淶���

��ϰ��ϵ�д�
�����Ŀ
ȡһ������NH4NO3�ͣ�NH4��2SO4��������ֳ�������ȵ����ȷݣ�һ����������NaOHŨ��Һ���ȣ��ڱ�״�����ռ���6.72L NH3����һ�ݼ�ˮ��ȫ�ܽ���������BaCl2��Һ�õ�11.65g��ɫ����������˵��������ǣ�������
| A��ԭ��������n[��NH4��2SO4]=0.05 mol |
| B��ԭ��������m��NH4NO3��=16 g |
| C������ȫ�ܽ����Һ�����Ϊ100 mL����c��NH4NO3��=4 mol?L-1 |
| D��ԭ��������n��NH4NO3����n[��NH4��2SO4]=4��1 |
X��Y��W��R��T��ǰ17��Ԫ���е�5�֣�X��Yλ��ͬ���壬Yԭ�ӵ������������������������ȣ�Rԭ�ӵ�����������Ϊ������������3����T�����ۣ�W����������������оƬ������˵������ȷ���ǣ�������
| A����̬�⻯���ȶ��ԣ�W��T |
| B�����Ӱ뾶��X��R |
| C������������Ӧˮ������ԣ�X��Y |
| D��Y����������ˮ���ҷ�Ӧ |
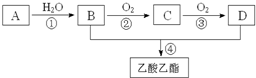 ��֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
��֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
