��Ŀ����
 ���������ʵ���г�������������KMnO4��Һ�����Ի���������·ֽ��ٶȽ���������������ˮ��Һ�ȶ��Խϲ�ж������̺���������������ֽ����ʼӿ죮
���������ʵ���г�������������KMnO4��Һ�����Ի���������·ֽ��ٶȽ���������������ˮ��Һ�ȶ��Խϲ�ж������̺���������������ֽ����ʼӿ죮��1�����������£�KMnO4��Һ������Ӧ�����ӷ���ʽ��
��2��ʵ���ҳ�
��3��ijͬѧ�����������Һ��εص���һ������IJ�������Һ�У����������������¶Ȳ��䣩�����ŵζ����̽��У�������ɫʱ���̣���ͬѧ��ΪH+�����뷴Ӧ�⣬�����˸÷�Ӧ��
��д���ζ��з�����Ӧ�����ӷ���ʽ
���������ͬѧ�����Ƿ���ȷ
��4���ۺ��������ۣ���ͬѧ�ⶨijKMnO4��Һ��Ũ�ȣ���
��һ�㳣ѡ��
���Լ����ƣ��У�
�ڵ�
| ʵ����� | KMnO4 ��Һ���/mL |
| I | 20.02 |
| II | 19.98 |
| III | 22.10 |
���㣺�к͵ζ�,����ʵ�鷽�������
ר�⣺ʵ����
��������1������Ŀ��Ϣ��֪��������������������»�ֽ����ɶ������̺�������ͬʱ������ˮ���ݴ���д���ӷ���ʽ��
��2�����Ը�����ؾ������Ժ�ǿ�����ԣ�����ʽ�ζ�����ȡ��
��3���ٸ������������Ʒ�Ӧ��MnO4-��Mn2+����Ԫ�ػ��ϼ���+7�۽���Ϊ+2�ۣ�������5�ۣ�C2O42-��CO2��̼Ԫ�ػ��ϼ���+3������Ϊ+4��������2�ۣ����ϼ���С������Ϊ10����MnO4-ϵ��Ϊ2��C2O42-ϵ��Ϊ5���ٸ���Ԫ���غ㡢����غ���ƽ����ʽ��
���ɷ�Ӧ����ʽ��֪������Ũ�ȼ�С������Ũ�ȶԷ�Ӧ���ʵ�Ӱ�������
��4��������ƽ��ȷ�Ƚϵͣ�Ӧ��ѡ�÷�����ƽ�������ƽ��
�����������ȶ����ܱ����������������������������ػ�ı���Ũ�ȣ�
�ڸ������Ϊ��ɫ������ζ��յ�ʱ��Һ����ɫ��Ϊdz��ɫ��
��5�����ݲ����Ƶ�������������ʵ�����Ũ�ȣ��ٸ��ݷ�Ӧ����ʽ�и������������Ƶ����ʵ�����ϵ���������ص����ʵ�����Ũ�ȣ�
��2�����Ը�����ؾ������Ժ�ǿ�����ԣ�����ʽ�ζ�����ȡ��
��3���ٸ������������Ʒ�Ӧ��MnO4-��Mn2+����Ԫ�ػ��ϼ���+7�۽���Ϊ+2�ۣ�������5�ۣ�C2O42-��CO2��̼Ԫ�ػ��ϼ���+3������Ϊ+4��������2�ۣ����ϼ���С������Ϊ10����MnO4-ϵ��Ϊ2��C2O42-ϵ��Ϊ5���ٸ���Ԫ���غ㡢����غ���ƽ����ʽ��
���ɷ�Ӧ����ʽ��֪������Ũ�ȼ�С������Ũ�ȶԷ�Ӧ���ʵ�Ӱ�������
��4��������ƽ��ȷ�Ƚϵͣ�Ӧ��ѡ�÷�����ƽ�������ƽ��
�����������ȶ����ܱ����������������������������ػ�ı���Ũ�ȣ�
�ڸ������Ϊ��ɫ������ζ��յ�ʱ��Һ����ɫ��Ϊdz��ɫ��
��5�����ݲ����Ƶ�������������ʵ�����Ũ�ȣ��ٸ��ݷ�Ӧ����ʽ�и������������Ƶ����ʵ�����ϵ���������ص����ʵ�����Ũ�ȣ�
���
�⣺��1������Ŀ��Ϣ��֪��������������������»�ֽ����ɶ������̺�������ͬʱ������ˮ����Ӧ���ӷ���ʽΪ��4MnO4-+4H+�T4MnO2��+3O2��+2H2O��
�ʴ�Ϊ��4MnO4-+4H+�T4MnO2��+3O2��+2H2O��
��2�����Ը�����ؾ������Ժ�ǿ�����ԣ��ܹ���ʴ��ʽ�ζ����е��ܣ���������ʽ�ζ�����ȡ����ѡ��B��
�ʴ�Ϊ��B��
��3���ٸ������������Ʒ�Ӧ����Ӧ��MnO4-��Mn2+����Ԫ�ػ��ϼ���+7�۽���Ϊ+2�ۣ�������5�ۣ�C2O42-��CO2��̼Ԫ�ػ��ϼ���+3������Ϊ+4��������2�ۣ����ϼ���С������Ϊ10����MnO4-ϵ��Ϊ2��C2O42-ϵ��Ϊ5���ٸ���Ԫ���غ��֪Mn2+ϵ��Ϊ2��CO2ϵ��Ϊ10�����ݵ���غ��֪H+ϵ��Ϊ16��������Ԫ���غ��֪H2Oϵ��Ϊ8����ƽ�����ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2+8H2O��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2+8H2O��
���ɷ�Ӧ����ʽ��֪������Ũ�ȼ�С����Ӧ����Ӧ�ü�С����ʵ���Ϸ�Ӧ��������˵�������Ӳ��Ǵ������ɷ�Ӧ�����Ƴ�����Ӧ����Mn2+��
�ʴ�Ϊ������ȷ���淴Ӧ���У�������Ũ����С����Ӧ�����Ӵ�˵�������Ӳ��Ǵ�����
��4��������ƽ��ȷ�Ƚϵͣ�ֻ��ȷ��0.1g������ 2.348g�����ƹ������250mL��Һ��Ӧ��ѡ�õ�����ƽ�������ƽ��
�ʴ�Ϊ��������ƽ�������ƽ��
�����������ȶ����ܱ������������������Ӧ��ѡ�������ữ����������������ػ�ı���Ũ�ȣ�����Ӧ�ð�����ӵ���������Һ�У�
�ʴ�Ϊ��H2SO4����������Һ��
�ڸ������Ϊ��ɫ������ζ��յ�ʱ��Һ����ɫ��Ϊdz��ɫ������Ҫ����Һ�ڰ�����ڲ���ɫ��
�ʴ�Ϊ����Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��
��5������ 2.348g�����ƹ������250mL��Һ��������Ƶ�Ũ��Ϊc�������ƣ�=
=0.0701mol/L��
������ʵ���������ϴ���ȥ����ǰ�������ĸ�����ص����Ϊ
=20.00ml��
12MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
12 5
0.02L��c 0.0701mol/L��0.025L
��c=
=0.21mol/L��
�ʴ�Ϊ��0.21mol/L��
�ʴ�Ϊ��4MnO4-+4H+�T4MnO2��+3O2��+2H2O��
��2�����Ը�����ؾ������Ժ�ǿ�����ԣ��ܹ���ʴ��ʽ�ζ����е��ܣ���������ʽ�ζ�����ȡ����ѡ��B��
�ʴ�Ϊ��B��
��3���ٸ������������Ʒ�Ӧ����Ӧ��MnO4-��Mn2+����Ԫ�ػ��ϼ���+7�۽���Ϊ+2�ۣ�������5�ۣ�C2O42-��CO2��̼Ԫ�ػ��ϼ���+3������Ϊ+4��������2�ۣ����ϼ���С������Ϊ10����MnO4-ϵ��Ϊ2��C2O42-ϵ��Ϊ5���ٸ���Ԫ���غ��֪Mn2+ϵ��Ϊ2��CO2ϵ��Ϊ10�����ݵ���غ��֪H+ϵ��Ϊ16��������Ԫ���غ��֪H2Oϵ��Ϊ8����ƽ�����ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2+8H2O��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2+8H2O��
���ɷ�Ӧ����ʽ��֪������Ũ�ȼ�С����Ӧ����Ӧ�ü�С����ʵ���Ϸ�Ӧ��������˵�������Ӳ��Ǵ������ɷ�Ӧ�����Ƴ�����Ӧ����Mn2+��
�ʴ�Ϊ������ȷ���淴Ӧ���У�������Ũ����С����Ӧ�����Ӵ�˵�������Ӳ��Ǵ�����
��4��������ƽ��ȷ�Ƚϵͣ�ֻ��ȷ��0.1g������ 2.348g�����ƹ������250mL��Һ��Ӧ��ѡ�õ�����ƽ�������ƽ��
�ʴ�Ϊ��������ƽ�������ƽ��
�����������ȶ����ܱ������������������Ӧ��ѡ�������ữ����������������ػ�ı���Ũ�ȣ�����Ӧ�ð�����ӵ���������Һ�У�
�ʴ�Ϊ��H2SO4����������Һ��
�ڸ������Ϊ��ɫ������ζ��յ�ʱ��Һ����ɫ��Ϊdz��ɫ������Ҫ����Һ�ڰ�����ڲ���ɫ��
�ʴ�Ϊ����Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��
��5������ 2.348g�����ƹ������250mL��Һ��������Ƶ�Ũ��Ϊc�������ƣ�=
| ||
| 0.25L |
������ʵ���������ϴ���ȥ����ǰ�������ĸ�����ص����Ϊ
| 19.98+20.02 |
| 2 |
12MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
12 5
0.02L��c 0.0701mol/L��0.025L
��c=
| 12��0.0701mol/L��0.025L |
| 5��0.02L |
�ʴ�Ϊ��0.21mol/L��
���������⿼�����к͵ζ�ʵ�顢���ӷ���ʽ����д��������ԭ��Ӧ�ζ�Ӧ�á���ѧ����ȣ���Ŀ�Ѷ��еȣ�ע�����ʵ���ԭ������Ҫѧ���߱�һ�������۷��������ͼ����������������

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
20�棬��֪2g������ȼ������ˮ����235kJ�������Ȼ�ѧ����ʽ��ȷ���ǣ�������
A��H2��g��+
| ||
| B��H2��g��+O2��g��=2H2O��1����H=-235kJ��mol-1 | ||
C��H2��g��+
| ||
| D��H2��g��+O2��g��=H2O��1����H=-470kJ��mol-1 |
��������ʢ�������������е������ǣ�������
| A��Ũ���� | B��Ũ���� |
| C������ͭ��Һ | D��ϡ���� |
�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������ý��ƫ�ͣ����������ԭ����������������еģ�������
| A���ζ������У�������ƽ�ӣ�ĩ�������� |
| B����ƿ������ˮϴ����δ�����T���еζ� |
| C����ʽ�ζ���δ�ñ�������ϴ |
| D���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ���ֹʱ������ʧ |
��˿��������ȼ�յ���Ҫ������FeCl3������FeCl2���Ⲣ����Ϊ��������
| A�������������Ժ�ǿ |
| B����ԭ�ӵĵõ���������ǿ |
| C���������Ժ�ǿ |
| D�������Ǻܻ��õķǽ��� |
��������Һ�У�Cl-���ʵ���Ũ����ͬ���ǣ�������
| A��1L 0.2 mol/L A1C13��Һ |
| B��100ml 0.3 mol/L MgCl2��Һ |
| C��0.5L 0.1 mol/L NaCl��Һ |
| D��1L 0.3 mol/L HC1��Һ |
 ��ҵ�ϡ��̶���������CO2����Ч�ؼ��ᡰ���ҡ�ЧӦ����һ����CO2�����״�ȼ�ϵķ�����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ?mol-1
��ҵ�ϡ��̶���������CO2����Ч�ؼ��ᡰ���ҡ�ЧӦ����һ����CO2�����״�ȼ�ϵķ�����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ?mol-1
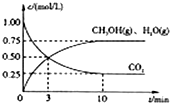 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�