��Ŀ����
17����1��CH3+��-CH3��������CH3-������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����CDE�����ţ���A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ�
C��CH3-��NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ�����
E������-CH3��������һ��CH3+��һ��CH3-��Ͼ��ɵõ�CH3CH3
��2��п��һ����Ҫ�Ľ�����п���仯�������Ź㷺��Ӧ�ã�
��п��Ԫ�����ڱ��е�λ���ǣ��������ڢ�B�壮
����������п[CH2OH��CHOH��4 COO]2Zn��Ŀǰ�г������еIJ�п����д��Zn2+��̬�����Ų�ʽ1s22s22p63s23p63d10�������Ƿ���[CH2OH��CHOH��4CHO]��̼ԭ���ӻ���ʽ��sp2��sp3�ӻ���
��Zn2+����NH3�γ�������[Zn��NH3��4]2+����λ��NH3�������ڼ��Է��ӣ�����Է��ӡ��Ǽ��Է��ӡ�������[Zn��NH3��4]2+�У�Zn2+λ�������������ģ�Nλ����������Ķ��㣬����ͼ1�б�ʾ��[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ����
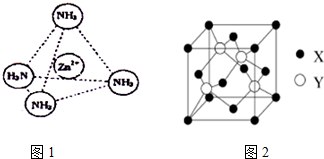
����ͼ2��ʾп��ij�ǽ���Ԫ��X�γɵĻ����ᄃ��������Zn��Xͨ�����ۼ���ϣ��û������Zn��X��ԭ�Ӹ���֮��Ϊ1��1��
���ڢܵľ�����ͼ2���У����ֻ����X�����з�ʽ����X�Ķѻ���ʽ���ڽ�������ѻ���ʽ�е����������ܶѻ��ѻ�����þ�����Zn�İ뾶Ϊr1 cm�������ԭ������ΪM1��X�İ뾶Ϊr2cm�������ԭ������ΪM2����þ�����ܶ�Ϊ$\frac{3\sqrt{3}��{M}_{1}+{M}_{2}��}{16{N}_{A}��{r}_{1}+{r}_{2}��^{3}}$g/cm3��д��������ĸ�ı���ʽ����
���� ��1��A������ȥ��һ����ԭ�Ӳ��ܵõ�CH3+��CH3-��
B��ԭ��������ȡ��۵����������������������ȵ�����Ϊ�ȵ����壻-CH3��������CH3-��Cԭ�Ӿ��γ�3���Ҽ�������̼ԭ����1�������ӣ�CH3-��Cԭ���ɶԹ¶Ե��ӣ��ӻ������Ŀ��Ϊ4��
C��CH3-��NH3��H3O+������4��ԭ�ӡ�10�����ӣ���Ϊ�ȵ����壬�ռ�ṹ���ƣ�
D��CH3+�е�̼ԭ���γ�3���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ3��Ϊƽ�������νṹ��
E������-CH3��һ��CH3+��CH3-��϶��ܵõ�CH3CH3��
��2����Znԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s2��
��Znԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s2��ʧȥ4s�ܼ�2�������γ�Zn2+��
�Ȼ���Cԭ���γ�3���Ҽ�������̼ԭ���γ�4���Ҽ�����û�й¶Ե��ӣ��ӻ������ĿΪ�ֱ�Ϊ3��4��
����λ��NH3����Ϊ�����νṹ������������������IJ��غϣ����ڼ��Է��ӣ�Zn2+�����пչ����NH3�����й¶Ե��ӣ�����ͨ����λ���γ�������[Zn��NH3��4]2+��
�ܸ��ݾ�̯�����㾧����Znԭ����Ŀ��Xԭ����Ŀ��
�ݾ�����X���ڶ��������ģ�Xԭ��Ϊ���������ܶѻ���
Znԭ������Χ��4��Xԭ�ӹ�����������ṹ������Znԭ���붥��Xԭ�����ߴ��ھ�����Խ����ϣ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$������Խ��߳���Ϊ4��r1+r2��cm���ʾ����ⳤΪ$\frac{4��{r}_{1}+{r}_{2}��}{\sqrt{3}}$cm����Ͼ����к���ԭ����Ŀ���㾧���������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺A��������ӱ��CH3+��-CH3��CH3-ʱ��ʧȥ�ķֱ����⸺���ӡ���ԭ�Ӻ������ӣ���A����
B��CH3+��-CH3��CH3-�ֱ����6����7����8���۵��ӣ����ǵȵ����壬-CH3��������CH3-��Cԭ�Ӿ��γ�3���Ҽ�������̼ԭ����1�������ӣ�CH3-��Cԭ���ɶԹ¶Ե��ӣ��ӻ������Ŀ��Ϊ4������̼ԭ�Ӳ�ȡsp3�ӻ�����B����
C��CH3-��NH3��H3O+������8���۵��ӡ�4��ԭ�ӣ���Ϊ�ȵ����壬���ι��;�Ϊ�����Σ���C��ȷ��
D��CH3+�е�̼ԭ���γ�3���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ3��̼ԭ�Ӳ�ȡsp2�ӻ���Ϊƽ�������νṹ������ԭ�Ӵ���ͬһƽ�棬��D��ȷ��
E������-CH3��һ��CH3+��CH3-��϶��ܵõ�CH3CH3����E��ȷ��
��ѡ��CDE��
��2����Znԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s2���������ڱ��е������ڢ�B�壬
�ʴ�Ϊ���������ڢ�B�壻
��Znԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s2��ʧȥ4s�ܼ�2�������γ�Zn2+��Zn2+��̬�����Ų�ʽΪ��1s22s22p63s23p63d10��
�Ȼ���Cԭ���γ�3���Ҽ�������̼ԭ���γ�4���Ҽ�����û�й¶Ե��ӣ��ӻ������ĿΪ�ֱ�Ϊ3��4��������̼ԭ���ӻ���ʽΪ��sp2��sp3�ӻ���
�ʴ�Ϊ��1s22s22p63s23p63d10��sp2��sp3��
����λ��NH3����Ϊ�����νṹ������������������IJ��غϣ����ڼ��Է��ӣ�Zn2+�����пչ����NH3�����й¶Ե��ӣ�����ͨ����λ���γ�������[Zn��NH3��4]2+����ͼ��ʾ��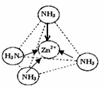 ��
��
�ʴ�Ϊ�����Է��ӣ� ��
��
�ܾ�����Znԭ����ĿΪ4��Xԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Zn��Xԭ����Ŀ֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
�ݾ�����X���ڶ��������ģ�Xԭ��Ϊ���������ܶѻ���
Znԭ������Χ��4��Xԭ�ӹ�����������ṹ������Znԭ���붥��Xԭ�����ߴ��ھ�����Խ����ϣ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$������Խ��߳���Ϊ4��r1+r2��cm���ʾ����ⳤΪ$\frac{4��{r}_{1}+{r}_{2}��}{\sqrt{3}}$cm����������Ϊ
$\frac{4����{M}_{1}+{M}_{2}��}{{N}_{A}}$g�����ܶ�=$\frac{4����{M}_{1}+{M}_{2}��}{{N}_{A}}$g�£�$\frac{4��{r}_{1}+{r}_{2}��}{\sqrt{3}}$cm��3=$\frac{3\sqrt{3}��{M}_{1}+{M}_{2}��}{16{N}_{A}��{r}_{1}+{r}_{2}��^{3}}$g/cm3��
�ʴ�Ϊ�����������ܶѻ���$\frac{3\sqrt{3}��{M}_{1}+{M}_{2}��}{16{N}_{A}��{r}_{1}+{r}_{2}��^{3}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų�ʽ���ռ乹�����ӻ���ʽ���жϡ��ȵ����塢������������ȣ������ܶȼ���Ϊ�״��㡢�Ѷȣ��ؼ��Ǽ��㾧���ⳤ����Ҫѧ���߱�һ���Ŀռ����������ݼ���������

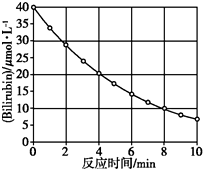 ������Bilirubin��һ�������Ĺ������·����ֽⷴӦ����Ӧ��Ũ���淴Ӧʱ��仯��ͼ��ʾ�����㷴Ӧ4��8 min���ƽ����Ӧ���ʺ��ƲⷴӦ16 minʱ��Ӧ���Ũ�ȣ����Ӧ�ǣ�������
������Bilirubin��һ�������Ĺ������·����ֽⷴӦ����Ӧ��Ũ���淴Ӧʱ��仯��ͼ��ʾ�����㷴Ӧ4��8 min���ƽ����Ӧ���ʺ��ƲⷴӦ16 minʱ��Ӧ���Ũ�ȣ����Ӧ�ǣ�������| A�� | 2.5 ��mol/�� L•min����2.0 ��mol/L | B�� | 2.5 ��mol/�� L•min����2.5 ��mol/L | ||
| C�� | 3.0 ��mol/�� L•min����3.0 ��mol/L | D�� | 5.0 ��mol/�� L•min����3.0 ��mol/L |
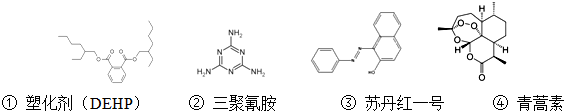
| A�� | �ܻ�����DEHP�����ӱ����ϵĶ��ȴ��������ֲ�ͬ�Ľṹ | |
| B�� | �����谷�ķ���ʽΪC3N6H6 | |
| C�� | �յ���һ�ŷ���������ԭ�ӿ�����ͬһƽ���� | |
| D�� | �������ܹ�����ű��������ṹ�д��ڹ����������й� |
| ѡ�� | ʵ�� | ���� | ���� |
| A | �ý��Q��˿պȡ��������Һ�ھƾ��ƻ��������� | ������ֻ�ɫ | ����Һ��ֻ��Na+������K+ |
| B | �������缫��ⱥ��ʳ��ˮ | �����Ͼ��������� | ���������ֱ�����H2��Cl2 |
| C | ����ˮ�м�������ʯ��ʯ | ������ɫ���� | ���ԣ�HCl0��H2CO3 |
| D | ��Ca��ClO��2��Һ�� ͨ����SO2 | ������ɫ���� | Ca��C1O��2���������ԣ�����ΪCaSO4 |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ����������ȼ�ϵ�ص���±ˮ����Cl-��Br-��Na+��Mg2+����װ����ͼ��ʾ��a��bΪʯī�缫��������˵����ȷ���ǣ�������
����������ȼ�ϵ�ص���±ˮ����Cl-��Br-��Na+��Mg2+����װ����ͼ��ʾ��a��bΪʯī�缫��������˵����ȷ���ǣ�������| A�� | ��ع���ʱ��B����ӦʽΪO2+2H2O+4e-=4OH- | |
| B�� | ���ʱ����������·���ǣ����������·����������Һ������������ | |
| C�� | �Թ���NaOH��Һ�������յ��ʱ������Cl2 | |
| D�� | �����������2.24L����״����H2ʱ��b����ΧҲ�����0.01mol���� |
| A�� | Ca2+ | B�� | OH- | C�� | Ba2+ | D�� | NH${\;}_{4}^{+}$ |
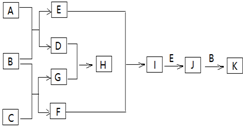 �й����ʵ�ת����ϵ��ͼ��ʾ��������������������ȥ������֪AΪ����ɫ���壬BΪ��������ɫҺ�壬CΪ����Ԫ����ɵĻ����Ħ������Ϊ150g/mol��EΪ�������壬FΪ��̬�⻯��г�������ζ��GΪ�����������KΪ�������ᣮ
�й����ʵ�ת����ϵ��ͼ��ʾ��������������������ȥ������֪AΪ����ɫ���壬BΪ��������ɫҺ�壬CΪ����Ԫ����ɵĻ����Ħ������Ϊ150g/mol��EΪ�������壬FΪ��̬�⻯��г�������ζ��GΪ�����������KΪ�������ᣮ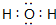 ��
�� ����
����
 ��F
��F ��
�� ����
���� ��
��