��Ŀ����
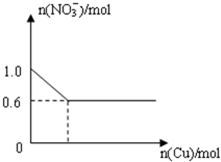 ��28.8gͭͶ��100mLŨ�����У����ͭ��ʣ�࣬��NO3-�������ʵ����仯��ͼ��ʾ����ش��������⣺
��28.8gͭͶ��100mLŨ�����У����ͭ��ʣ�࣬��NO3-�������ʵ����仯��ͼ��ʾ����ش��������⣺��1����ʼ��ӦʱŨ��������ʵ���Ũ��Ϊ
��2��ͭ��Ũ���ᷴӦ���ɵ������ڱ�״���µ������������NO2��N2O4��ƽ�⣩Ϊ
��3��������ȫ��Ӧ������ͭ�����ʵ���n��Cu����
��4��Ӧ�����˷�Ӧ��ϵ�еμ�
��5��a gͭȫ������һ������Ũ�����У�������ɵ������ڱ�״���µ������������NO2��N2O4��ƽ�⣩Ϊb L��Ϊ������Ⱦ�������ɵ�����ͨ��NaOH��Һ�У����屻��ȫ���գ�
��֪��NO+NO2+2NaOH��2NaNO2+H2O 2NO2+2NaOH��NaNO3+NaNO2+H2O
���NO��NO2���������NOx��ʾ����NaOH��Һ��ȫ����ʱ��x��ȡֵ��Χ
��6���ڳ����£���NO����ѹ����1.01��107Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
| 2 |
| 3 |
���㣺��ѧ����ʽ���йؼ���
ר�⣺������
��������1�����ݿ�ʼʱ����������ʵ������������Ũ�ȣ�
��2�����õ�Ԫ���غ������������ʵ������ٸ���V=nVm������������������
��3����Ӧ��������Һ������Ϊ����ͭ�������������������n��Cu����
��4��������Ӧ��3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O���ݴ˼���������������ʵ���������������Ҫ����������
��5���ɷ���ʽ2NO2+2NaOH=NaNO2+NaNO3+H2O��NO+NO2+2NaOH=2NaNO2+H2O��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С������x����Сֵ����Ϊ����NO������x���ֵ��2���ݴ�ȷ��xȡֵ��Χ��
�ݹ��������̣�Cuʧȥ�ĵ��ӵ�������������������ʱ��õĵ��ӣ����ݵ���ת���غ����n��NaNO2��������NԪ���غ����n��NaNO3����
��6���ڳ����£���NO����ѹ����1.01��107Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
��Ȼ��ѹǿ�Ͳ��ٸı䣮��֪����һ�ֲ���ΪN2O������ԭ�Ӹ����غ��֪�÷�ӦΪ��3NO=N2OʮNO2����Ϊ����2NO2 N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
��
��ֻ������Ӧ��3NO=N2OʮNO2�������ƽ��Ħ��������С��������������ȫת��Ϊ����������ʱ�������ƽ��Ħ��������ݴ˼�����
��2�����õ�Ԫ���غ������������ʵ������ٸ���V=nVm������������������
��3����Ӧ��������Һ������Ϊ����ͭ�������������������n��Cu����
��4��������Ӧ��3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O���ݴ˼���������������ʵ���������������Ҫ����������
��5���ɷ���ʽ2NO2+2NaOH=NaNO2+NaNO3+H2O��NO+NO2+2NaOH=2NaNO2+H2O��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С������x����Сֵ����Ϊ����NO������x���ֵ��2���ݴ�ȷ��xȡֵ��Χ��
�ݹ��������̣�Cuʧȥ�ĵ��ӵ�������������������ʱ��õĵ��ӣ����ݵ���ת���غ����n��NaNO2��������NԪ���غ����n��NaNO3����
��6���ڳ����£���NO����ѹ����1.01��107Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
| 2 |
| 3 |
 N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��| 2 |
| 3 |
��ֻ������Ӧ��3NO=N2OʮNO2�������ƽ��Ħ��������С��������������ȫת��Ϊ����������ʱ�������ƽ��Ħ��������ݴ˼�����
���
�⣺��1����ͼ��֪����ʼʱn��NO3-��=1.0mol����n��HNO3��=n��NO3-��=1.0mol����c��HNO3��=
=10mol/L���ʴ�Ϊ��10��
��2����ͼ��֪����Ӧ����ʱ����Һ��NO3-Ϊ0.6mol�����ݵ�Ԫ���غ㣬������������ʵ���Ϊ1.0mol-0.6mol=0.4mol������¶������������Ϊ0.4mol��22.4L/mol=8.96L���ʴ�Ϊ��8.96��
��3����Һ������Ϊ����ͭ����ͼ��֪����Ӧ����ʱ����Һ��NO3-Ϊ0.6mol��������n��Cu��=n������ͭ��=
=0.3mol���ʴ�Ϊ��0.3��
��4��28.8gͭ�����ʵ���Ϊ
=0.45mol����ʣ��CuΪ0.45mol-0.3mol=0.15mol��
3 Cu+2 NO3-+8 H+=3Cu2++2NO��+4H2O
3 2 8
0.15mol n��H+��
��n��H+��=
=0.4mol����n��H2SO4��=0.2mol������Ҫ�������Ϊ
=0.1L=100mL��
�ʴ�Ϊ��100��
��5���ɷ���ʽ��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С��x��СֵΪ
=1.5����Ϊ����NO������x���ֵ��2����x��ȡֵ��ΧΪ1.5��x��2��
�ݹ��������̣�Cuʧȥ�ĵ��ӵ�������������������ʱ��õĵ��ӣ����ݵ���ת���غ㣬n��NaNO2��=n��Cu��=
=
mol������NԪ���غ㣬��֪n��NaNO3��=n�����壩=
-
mol=��
-
��mol��
�ʴ�Ϊ��1.5��x��2��
����
-
����
��6���ڳ����£���NO����ѹ����1.01��107Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
��Ȼ��ѹǿ�Ͳ��ٸı䣮��֪����һ�ֲ���ΪN2O������ԭ�Ӹ����غ��֪�÷�ӦΪ��3NO=N2OʮNO2����Ϊ����2NO2 N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
��
��ֻ������Ӧ��3NO=N2OʮNO2�������ƽ��Ħ��������С����ʱM=
=45g/mol��
������������ȫת��Ϊ����������ʱ�������ƽ��Ħ���������1molNO2�õ�0.5molN2O4��
���ʱM=
=60g/mol��
���Ϸ�������֪45g/mol��M��60g/mol��
�ʴ�Ϊ��3NO=N2OʮNO2��2NO2 N2O4��45g/mol��M��60g/mol��
N2O4��45g/mol��M��60g/mol��
| 1.0mol |
| 0.1L |
��2����ͼ��֪����Ӧ����ʱ����Һ��NO3-Ϊ0.6mol�����ݵ�Ԫ���غ㣬������������ʵ���Ϊ1.0mol-0.6mol=0.4mol������¶������������Ϊ0.4mol��22.4L/mol=8.96L���ʴ�Ϊ��8.96��
��3����Һ������Ϊ����ͭ����ͼ��֪����Ӧ����ʱ����Һ��NO3-Ϊ0.6mol��������n��Cu��=n������ͭ��=
| 0.6mol |
| 2 |
��4��28.8gͭ�����ʵ���Ϊ
| 28.8g |
| 64g/mol |
3 Cu+2 NO3-+8 H+=3Cu2++2NO��+4H2O
3 2 8
0.15mol n��H+��
��n��H+��=
| 0.15mol��8 |
| 3 |
| 0.2mol |
| 2mol/L |
�ʴ�Ϊ��100��
��5���ɷ���ʽ��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С��x��СֵΪ
| 2+1 |
| 2 |
�ݹ��������̣�Cuʧȥ�ĵ��ӵ�������������������ʱ��õĵ��ӣ����ݵ���ת���غ㣬n��NaNO2��=n��Cu��=
| ag |
| 64g/mol |
| a |
| 64 |
| bL |
| 22.4L/mol |
| a |
| 64 |
| b |
| 22.4 |
| a |
| 64 |
�ʴ�Ϊ��1.5��x��2��
| a |
| 64 |
| b |
| 22.4 |
| a |
| 64 |
��6���ڳ����£���NO����ѹ����1.01��107Pa���ټ��ȵ�50�棬���������ѹǿѸ���½���ѹǿ������С��ԭѹǿ��
| 2 |
| 3 |
 N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��
N2O4�������ɵ�NO2��˫�۳�N2O4������������������٣�ѹǿ������С��ԭѹǿ��| 2 |
| 3 |
��ֻ������Ӧ��3NO=N2OʮNO2�������ƽ��Ħ��������С����ʱM=
| 3��30g/mol |
| 2 |
������������ȫת��Ϊ����������ʱ�������ƽ��Ħ���������1molNO2�õ�0.5molN2O4��
���ʱM=
| 1mol��44g/mol+0.5mol��92g/mol |
| 1mol+0.5mol |
���Ϸ�������֪45g/mol��M��60g/mol��
�ʴ�Ϊ��3NO=N2OʮNO2��2NO2
 N2O4��45g/mol��M��60g/mol��
N2O4��45g/mol��M��60g/mol��
���������⿼�黯ѧ���㣬����ƴ������Ŀ���漰�غ㷨�����������õȣ��Ƕ�ѧ���ۺ������Ŀ��飬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ�����˵������ȷ���ǣ�������
| A����������Ԫ�ش�Na��Clԭ�Ӱ뾶��С |
| B������Ԫ��ȫ�ǽ���Ԫ�أ�������Ѱ���������� |
| C��ͬһ����Ԫ�صĵ��ʣ����ϵ��»�ѧ����Խ��Խ���� |
| D��������������Ԫ�ص���������ϼ��������������������� |
��ʵ�����У����г��ӵķ�������ȷ���ǣ�������
| A���屽�л����壬����KI��Һ������������ȡ���� |
| B�������л�����ϩ��ͨ��H2��һ�������·�Ӧ��ʹ��ϩת��Ϊ���� |
| C���������л���Ũ�����Ũ���ᣬ���䵹��NaOH��Һ�У����ã���Һ |
| D����ϩ�л���CO2��SO2������ͨ��NaOH��Һ��ϴ�� |
ʹ�ò��������ܴﵽ����Ŀ���ǣ�������
| A������ |
| B����ֹҺ�彦�� |
| C���������ʵ��ܽ�� |
| D���ӿ����ʵ��ܽ��ٶ� |
��þ���Ͻ������Ĵ������š�����������Ʒ���ɡ����ۡ����ã���������Щ�ص��ص�þ���Ͻ�������ǣ�������
| A���������� | B�������Ժ� |
| C���ܶ�С | D��ǿ�ȸ� |
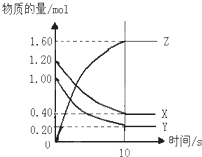 ����ͼ��ʾ��ij�¶��£����ʵ����ֱ���1.2mol������X�����ʵ���Ϊ1.0mol������Y����2L�ܱ������з�Ӧ��������Z����Ӧ5min����n��X��=0.4mol��n��Y��=0.2mol�����ɵ�n��Z��=1.6mol����÷�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ
����ͼ��ʾ��ij�¶��£����ʵ����ֱ���1.2mol������X�����ʵ���Ϊ1.0mol������Y����2L�ܱ������з�Ӧ��������Z����Ӧ5min����n��X��=0.4mol��n��Y��=0.2mol�����ɵ�n��Z��=1.6mol����÷�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ