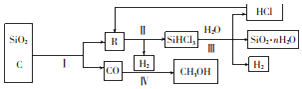
��Ŀ����
13��ijѧϰС�鰴��ͼʵ������̽峺����е⺬���IJⶨ�͵����ȡ��
��֪����֪��3I2+6NaOH�T5NaI+NaIO3+3H2O ��ش�
��1��ʵ�������������ƣ�A������B500 mL����ƿ��
��2������ڵIJ���������ͬ�����ͬ������ͬ����
��3������XΪ��ԭҺ�еμ�0.0100mol/LAgNO3��Һ��������ԭҺ�в������ɳ���ʱ������ AgNO3��Һ20.00mL������ú����е�İٷֺ���Ϊ1.27%��
��4������Y�У���ȡ���Һ©���ڹ۲쵽��������Һ���Ϊ�������㣬�²���Ϻ�ɫ��
��5���������в����������������ǵ����������ᵼ�µ����ʧ��
��6�������йز���Z��˵������ȷ����AB��
A��Ӧ����NaOH��Һ��Ũ�Ⱥ���� B������ת�������ӽ���ˮ��
C��NaOH��Һ�������Ҵ����森
���� �Ժ���Ϊԭ��̽峺����е⺬���IJⶨ�ͻ�������ⵥ�ʣ�������A���������պ������õ������ң��������ҽ��ݵõ�����������Һ��Ȼ����ù��˵ķ�������������Һ���룬�õ����е⻯�ص���Һ����ˮ��Һ�м������������γɵ⻯�����������ݵ��غ����ú����е�İٷֺ�����
����ȡҺ��ͨ������������ҺPH��4-5���õ���I2��ˮ��Һ�������л��ܼ����Ȼ�̼��ȡ��Һ�õ���I2��CCl4��Һ���������ǣ�����Y��������������Һ���շ�Һ�õ��ϲ���Һ����NaI��NaIO3������H2SO4��Һ�������з�Ӧ�õ�I2��ˮ��Һ�����˵õ��ֵ������ᴿ�õ������ⵥ�ʣ���������ֱ������õ��ⵥ�ʣ�
��1�����չ�����Ҫʹ������������������Һ���Ϊ500mL��Ӧ��ѡ�ù��Ϊ500mL������ƿ��
��2���������ҽ��ݵõ�����������Һ��������Ϊ���ˣ�����������Һ���룬I2��ˮ��Һ�������ڹ��˵õ��ֵ⣻
��3������������������ӵķ�Ӧ�����100mL��Һ�к��е����ӵ����ʵ������ټ����500mL��Һ�к��еĵ����ӣ�������������е�İٷֺ�����
��4���ⵥ���������л��ܼ��������Ȼ�̼��Һ�ܶȴ���ˮ��Һ������ж���ȡ����
��5���ӵⵥ���������Ƕȷ�����
��6��A����Ӧ3I2+6NaOH�T5NaI+NaIO3+3H2O�У���ҪŨ����������Һ��
B���ⵥ�����������Ʒ�Ӧ�����˵⻯�ơ������ƣ�
C���Ҵ�������ˮ�����Ȼ�̼����Ȼ��������ⵥ�ʣ�
��� �⣺��1�����չ�������һ��ʹ�ã��ɣ����������Բ�������պ���ʱ�������������н��У�ͨ������B����500mL���е����ӵĽ�ȡҺ����Ҫʹ��500mL������ƿ��
�ʴ�Ϊ��������500 mL����ƿ��
��2���Ժ���Ϊԭ��̽峺����е⺬���IJⶨ�ͻ�������ⵥ�ʣ�������A���������պ������õ������ң��������ҽ��ݵõ�����������Һ��Ȼ����ù��˵ķ�������������Һ���룬���Բ�����Ϊ���ˣ�����ȡҺ��ͨ������������ҺPH��4-5���õ���I2��ˮ��Һ�������л��ܼ����Ȼ�̼��ȡ��Һ�õ���I2��CCl4��Һ���������ǣ�����Y��������������Һ���շ�Һ�õ��ϲ���Һ����NaI��NaIO3������H2SO4��Һ�������з�Ӧ�õ�I2��ˮ��Һ�����˵õ��ֵ������ᴿ�õ������ⵥ�ʣ����Բ�����ҲΪ���ˣ�����ڵIJ���������ͬ��
�ʴ�Ϊ����ͬ��
��3��20.00mL��������Һ�к��������������ʵ���Ϊ��0.0100mol/L��0.02L=0.0002mol����500mLԭ����Һ��ȫ��Ӧ���������������ʵ���Ϊ��0.0002mol��$\frac{500mL}{50mL}$=0.002mol��
˵��20.00g�ú����к���0.002mol�����ӣ����Ժ����е�İٷֺ���Ϊ��$\frac{127g/mol��0.002mol}{20.00g}$��100%��1.27%��
�ʴ�Ϊ��1.27%��
��4���ⵥ���������л��ܼ�������ˮ�������Ȼ�̼���ܶȴ���ˮ��Һ�����Բ���Y�У����еⵥ�ʵ�ˮ��Һ�м������Ȼ�̼���Һ���Ϊ�������㣬�²�Ϊ���Ȼ�̼�ĵ���Һ�����²���Ϻ�ɫ��
�ʴ�Ϊ��Һ���Ϊ�������㣬�²���Ϻ�ɫ��
��5���������в���������������ڵⵥ�������������ᵼ�µⵥ����ʧ�����Լ�����������
�ʴ�Ϊ�������������ᵼ�µ����ʧ��
��6��A��������Ӧ3I2+6NaOH�T5NaI+NaIO3+3H2O�У���ҪŨ����������Һ������Ӧ����NaOH��Һ��Ũ�Ⱥ��������A��ȷ��
B�����ݷ�Ӧ3I2+6NaOH�T5NaI+NaIO3+3H2O��֪������Y����ת�������ӽ���ˮ�㣬��B��ȷ��
C���Ҵ�������ˮ�����Ȼ�̼�����������ƻ����Ҵ�����Ȼ��������ⵥ�ʣ���C����
�ʴ�Ϊ��AB��
���� ���⿼�����ʵķ������ᴿ�������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ֿ���ѧ���ķ�����������������ѧʵ����������ȷʵ��ԭ��Ϊ���ؼ���ע���������ջ�ѧʵ���������������

 һ����ʦȨ����ҵ��ϵ�д�
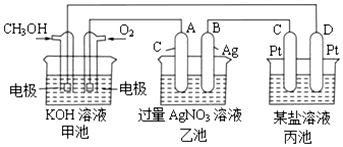
һ����ʦȨ����ҵ��ϵ�д� ������ҹ��ز������Ҫ�ɷ֣��ṹ��ͼ��ʾ��������������Ϊ��״��������˵��������ǣ�������
������ҹ��ز������Ҫ�ɷ֣��ṹ��ͼ��ʾ��������������Ϊ��״��������˵��������ǣ�������| A�� | ����FeCl3��Һ������ɫ��Ӧ | |
| B�� | ��ʹ����KMnO4��Һ��ɫ | |
| C�� | ����NaHCO3��Һ��Ӧ�ų�CO2 | |
| D�� | 1mol���������Ũ��ˮ��Ӧ�������5molBr2 |
| A�� | C15H22O5 | B�� | C15H20O5 | C�� | C15H18O5 | D�� | C15H24O5 |
 +
+ $��_{75-80��}^{H_{3}PO_{4}}$
$��_{75-80��}^{H_{3}PO_{4}}$ +CH3COOH
+CH3COOHΪ���������ˮ�⣬����ԭ�ϡ�����Ҫ��ָ���й����ʵIJ��������
| ���� | ʽ�� | ���� | �۵�/�� | �е�/�� | �ܽ�� | ||
| ˮ | �Ҵ� | �������� | |||||
| ˮ���� | 138 | ��ɫ�ᾧ��ĩ���� | 157��159 | 211 | �� | ���� | ���� |
| ������ | 102 | ��ɫҺ�塢��ȼ���д���ζ | -73.1 | 138.6 | ���� | �� | ���� |
| ����ˮ���� | 180 | ��ɫ��״�ᾧ | 135 | 321.4 | ��ˮ�ܡ���ˮ���� | ���� | �� |

�ش��������⣺
��1��Ũ����������Ǵ���������ڵļ��ȷ�ʽΪˮԡ���ȣ��¶ȿ�����75��80�森
��2����ֲ����м��������������������ܽ�ˮ����ʹ����������ʣ�ͬʱ��������ˮ�������ܽ����ɵ���ʧ��
��3����֤���õ�������ˮ�����Ʒ��Ϊ�����ķ����Dzⶨ��Ʒ�۵㣮
��4�����ƺ�����ˮ���������Ϊ8.91g���������Ϊ66%����ɲ��ʵ͵�ԭ������з�Ӧ�¶�û�п��ƺã���ֲ�Ʒ����ʱ���¶ȹ��ߣ���ɲ�Ʒˮ�ⷢ������Ӧ�ȣ�����дһ�㣩��
| A�� | NaCl���������ӻ�������м��Լ� | |
| B�� | H2O����1mol�к���2mol�Ǽ��Լ� | |
| C�� | KOH���Ⱥ������Ӽ����ֺ��м��Թ��ۼ� | |
| D�� | NaNO3�����Ǻ������Ӽ��Ĺ��ۻ����� |
| A�� | ԭ����Դ�� | B�� | ��ȼ�գ��ų������� | ||
| C�� | ��������Ⱦ | D�� | ��ȡH2������ |
| A�� | 5.6g | B�� | 11.2g | C�� | 16.8g | D�� | 22.4g |