��Ŀ����
4������ʵ���к죬��Ӧ��ʵ�������Լ����۶���ȷ�����߾��������ϵ���ǣ�������| ѡ�� | ʵ�� | ���� | ���� |
| A | ��pH��ֽ�ⶨ��Ũ�ȵ�HCl��H2SO4����Һ��pH | �ⶨHCl��Һ��pH��ֽ���� | �ǽ�����Cl��S |
| B | ��AgCl��AgBr�ı�����Һ�������� | ���ֵ���ɫ���� | Ksp��AgBr����Ksp��AgCl�� |
| C | ��Al2��SO4��3��Һ�еμӹ�������������Һ | ���ɰ�ɫ���� | A l��OH��3 ����������������Һ |
| D | ��Fe��NO3��2 ��Ʒ����ϡ���ᣬ�μ�KSCN��Һ | ��Һ��� | Fe��NO3��3 ��Ʒ����ϡ����ǰ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��Ӧ������������ˮ��������ԱȽϷǽ����ԣ�
B�����ֵ���ɫ������˵��AgI���ܽ�Ƚ�С��
C����Ӧ�������ᱵ������
D�����������£���������Ӿ���ǿ�����ԣ�
��� �⣺A��ʵ��ֻ��˵��������������Ũ�Ƚϴ��ܱȽ�����ǿ���Լ��ǽ����ԣ�Ӧ������������ˮ��������ԱȽϷǽ����ԣ���A����
B�����ֵ���ɫ������˵��AgI���ܽ�Ƚ�С����˵��Ksp��AgBr����Ksp��AgCl������B��ȷ��
C����Ӧ�������ᱵ����������˵���������������Ƿ��ܽ⣬��C����
D�����������£���������Ӿ���ǿ�����ԣ�����֤���Ƿ���ʣ���D����
��ѡB��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ�����ʵ��������ע��������ʵ������Լ�ʵ��������ԺͿ����Ե����ۣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14�����б仯�У�һ��Ҫ�����ʵ��Ļ�ԭ������ʵ�ֵ��ǣ�������
| A�� | KMnO4��MnO2 | B�� | HCl��Cl2 | C�� | Fe2O3��Fe | D�� | CaO��CaCO3 |
15������ȷ��ʾ���з�Ӧ�����ӷ���ʽ���ǣ�������
| A�� | ����ˮ��Ӧ��2Na+2H2O�T2Na++2OH-+H2�� | |
| B�� | ����CuSO4��Һ��Ӧ��2Na+Cu2+�TCu+2Na+ | |
| C�� | �������ᷴӦ��2Na+2H2O�T2Na++2OH-+H2�� | |
| D�� | ��������������Һ��Ӧ��2Al+2OH-+2H2O�T2AlO2-+H2�� |
12�������йص������Һ�У����ʵ���Ũ�ȹ�ϵ������ǣ�������
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ����Ȼ�ϣ�c��H+��+c��M+ ��=c��OH- ��+c��A- �� | |
| B�� | 25��ʱ��pH=4.7Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ�У���CH3COO-��+c��OH-����c��CH3COOH��+c��H+�� | |
| C�� | ����������Ա�̼��������NaHS��Һ�У�c��Na+����c��HS-����c��H+����c��OH-�� | |
| D�� | ��0.2mol•L-1NH4Cl��Һ��0.1mol•L-1��NH4��2Fe��SO4��2��Һ��0.2mol•L-1NH4HSO4��Һ��0.1mol•L-1��NH4��2CO3��Һ�У�c��NH4+ ����С���ۣ��ڣ��٣��� |
19��������Һ��Cl-Ũ�������ǣ�������
| A�� | 800mL0.5mol/L��NaCl��Һ | B�� | 100mL0.3mol/L��AlCl3��Һ | ||
| C�� | 500mL0.3mol/L��CaCl2��Һ | D�� | 300mL0.3mol/L��MgCl2��Һ |
9��X��Y��Z��W������ԭ��������������Ķ�����Ԫ�أ���֪��X��Wλ��ͬһ���壬��W��ԭ��������X��2����Z��ͬ�����м����Ӱ뾶��С��Ԫ�أ�Y��ԭ��������ZСl������˵������ȷ���ǣ�������
| A�� | Y��Z�Ż�ʱ��������ĭ������������ | |
| B�� | ����������Ӧˮ����ļ��ԣ�Y��Z | |
| C�� | Y��Z��W�ļ����Ӷ��ܴٽ�ˮ�ĵ��� | |
| D�� | ԭ�Ӱ뾶��С�����˳��X��W��Z��Y |
6�������Ϊ2L�ĺ����ܱ������м���4molH2��һ������CO������Ӧ��CO��g��+2H2��g��?CH3OH��g����H��0��CO��CH3OH ��g�������ʵ�����ʱ��ı仯���±���ʾ��������˵��������ǣ�������
| t/min | 0 | 3 | 10 | 12 |
| n��CO��/mol | 2 | 1 | 0.5 | 0.5 |
| n��CH3OH��/mol | 0 | 1 | 1.5 | 1.5 |
| A�� | ��0��3min�ڣ���H2��ʾ��ƽ����Ӧ����Ϊ0.33mol•L-1•min-1 | |
| B�� | �ڸ������£�������Ӧ��ƽ�ⳣ��Ϊ3 | |
| C�� | ��Ӧ��ƽ��ʱ��CH3OH ��g�����������Ϊ50% | |
| D�� | Ҫ����Ӧ���������� CH3OH ��g���ڻ�����е�����������ɲ���ѹ�������������ϵѹǿ�Ĵ�ʩ |
7��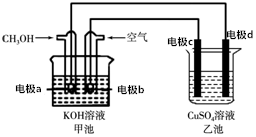 �ø�װ�ý��д�ͭ��������ͭ��һ�㺬��п����������������ʣ������в������������ʣ�����ͼ��ʾ��װ���У���ͨ��·һ��ʱ�������һ���缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������
�ø�װ�ý��д�ͭ��������ͭ��һ�㺬��п����������������ʣ������в������������ʣ�����ͼ��ʾ��װ���У���ͨ��·һ��ʱ�������һ���缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������
 �ø�װ�ý��д�ͭ��������ͭ��һ�㺬��п����������������ʣ������в������������ʣ�����ͼ��ʾ��װ���У���ͨ��·һ��ʱ�������һ���缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������
�ø�װ�ý��д�ͭ��������ͭ��һ�㺬��п����������������ʣ������в������������ʣ�����ͼ��ʾ��װ���У���ͨ��·һ��ʱ�������һ���缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������| A�� | �׳ػ�ѧ��ת��Ϊ���ܣ�����ʱOH-��a��b�����ƶ� | |
| B�� | ͨ��ʱ��������·�е�������Ϊb��c��d��a | |
| C�� | �ҳ���CuSO4��Һ��Ũ�Ȳ��� | |
| D�� | �缫d�Ǵ�ͭ���˹����е���ת��0.05mol |