��Ŀ����
20�� ʵ�����Ʊ����״��ͱ�����Ļ�ѧԭ����
ʵ�����Ʊ����״��ͱ�����Ļ�ѧԭ����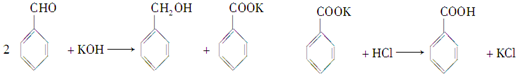
��֪����ȩ�ױ��������������״��ķе�Ϊ205.3�棻��������۵�Ϊ121.7�棬�е�Ϊ249�棬�ܽ��Ϊ0.34g�����ѵķе�Ϊ34.8�棬������ˮ���Ʊ����״��ͱ��������Ҫ�������£�
����ȩ$��_{����24h}^{KOH��H_{2}O}$��ɫ��״��$��_{������}^{ˮ������}$
�Ը���������Ϣ�ش��������⣺
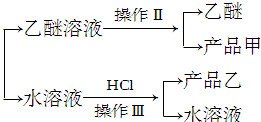
��1�����������������ȡ����Һ����������Һ��������Ҫ�ɷ��DZ��״���
��2�������������������Ʒ���DZ��״���
��3��������������ǹ��ˣ���Ʒ���DZ����ᣮ
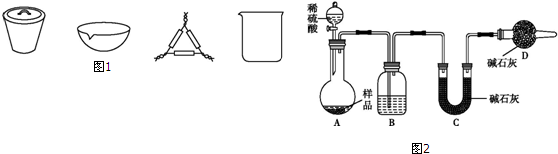
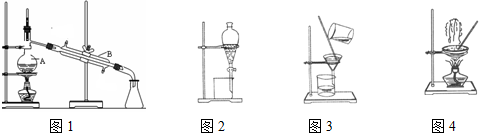
��4����ͼ��ʾ�����������¶ȼ�ˮ�������ط��õ�λ��Ӧ��b���a������b������c����d�������ò����У�����������ƿ���¶ȼ��⣬����Ҫ�IJ��������������ܡ��ƾ��ơ���ƿ��ţ�ǹܣ��ռ���Ʒ���ѵ������¶�Ϊ34.8�森
���� �����̽��������Ϣ��֪������ȩ��KOH��Ӧ���ɰ�ɫ��״��Ϊ���״���������صĻ���Ȼ���ˮ��������ȡ���״�����������Һ�к����״�������IIΪ���õ���Ʒ��Ϊ���״���ˮ��Һ�к�������أ������ᷢ��ǿ����ȡ����ķ�Ӧ�����ɱ����ᣬ��������ܽ��С���������Ϊ���ˣ����Ʒ��Ϊ�����ᣬ�Դ˼��Խ��
��� �⣺�����̿�֪������ȩ��KOH��Ӧ���ɱ��״���������أ�Ȼ���ˮ��������ȡ���״�����������Һ�к����״�������IIΪ���õ���Ʒ��Ϊ���״���ˮ��Һ�к�������أ������ᷢ��ǿ����ȡ����ķ�Ӧ�����ɱ����ᣬ��������ܽ��С���������Ϊ���ˣ����Ʒ��Ϊ�����ᣬ
��1��������������������Ϊ��ȡ����Һ��������Һ��������Ҫ�ɷֱ��״���
�ʴ�Ϊ����ȡ����Һ�������״���
��2��������Һ�к����״�������IIΪ���õ���Ʒ��Ϊ���״���
�ʴ�Ϊ�������״���
��3��ˮ��Һ�к�������أ������ᷢ��ǿ����ȡ����ķ�Ӧ�����ɱ����ᣬ��������ܽ��С���������Ϊ���ˣ����Ʒ��Ϊ�����ᣬ
�ʴ�Ϊ�����ˣ������
��5������ʱ���¶ȼƵ�ˮ����Ӧ��֧�ܿڣ����¶ȼ�ˮ����x�ķ���λ��Ϊb��
����ʵ������Ҫ�IJ��������У�������ƿ���¶ȼơ������ܡ�ţ�ǹܣ�β�ӹܣ����ƾ��ơ���ƿ�����Ի�ȱ�ٵIJ�������Ϊ�������ܡ��ƾ��ơ���ƿ��ţ�ǹܣ�
ͨ�����������������ѣ��������ѵķе��֪�����������¶�Ϊ34.8�棬
�ʴ�Ϊ��b�������ܡ��ƾ��ơ���ƿ��ţ�ǹܣ�34.8�森
���� ���⿼���Ʊ���������ƣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ���ȷ�л�������ʼ����������еķ�Ӧ���������뷽��Ϊ���Ĺؼ���ע�������Ʊ�ʵ�鷽�������ԭ���ط�����ʵ�������Ŀ��飮

| A�� | ���� | B�� | ������ | C�� | ���ܼ� | D�� | ��ǿ�� |
| A�� | ������ˮ | B�� | ������ˮ | C�� | ��ˮ | D�� | ��ˮ |
| A�� | �������ķ������������������� | |
| B�� | SiO2��NaCl��I2��C2H6O������ʵ��ʾ���ʷ������ | |
| C�� | ����̪ݼ�����ӣ�ֱ��Ϊ1.3��10-9�ף���ˮ���γɵķ�ɢϵ�ܲ��������ЧӦ | |
| D�� | �����ᡢ���ȼ������ᱵ��SO2�ֱ������ᡢ����ǿ����ʡ��ǵ���� |
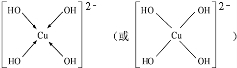
 ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ�
��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ� ��C���⻯����B���⻯����ȶ���ǿ��˳��ΪHF��H2S���ѧʽ����
��C���⻯����B���⻯����ȶ���ǿ��˳��ΪHF��H2S���ѧʽ���� ��
��