��Ŀ����
17����ͼ��ʾ����װ����XΪ�����ӽ���Ĥ�����ѵĽṹ��ʽΪCH3OCH3��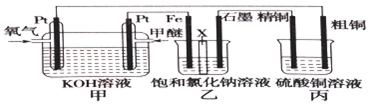
����Ҫ��ش�����������⣺
��1��д�������ĵ缫��Ӧ��CH3OCH3-12e-+16OH-�T2CO32-+11H2O��
��2������������Ҫ�����������������ʯī�����������ɣ�
��3�������ͭ�к���п���������ʣ����ͭ�ĵ缫��ӦΪ��Zn-2e-=Zn2+��Cu-2e-=Cu2+��
��4����Ӧһ��ʱ�䣬����ͭ��Һ��Ũ�Ƚ���С�����������С�����䡱����
��5�����ڱ�״������2.24L�����μӷ�Ӧ����װ������������ͭ������Ϊ12.8g��
��6��������װ�øij��ڱ�״���£���ʯī���缫������Ϊ2L��CuSO4��Һ��д�����CuSO4��Һʱ�����ӷ�Ӧ����ʽ2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+��������ʵ�ʱ���Ͽ���Դ������ֻ�貹��22.2g����Cu2��OH��2CO3����ʹ���Һ�ָ���ԭŨ�����������ԭCuSO4��Һ��Ũ����0.1mol/L����������Ƴ��ڶƼ��϶�ͭ��װ�ã�����θĶ������ü�Ҫ��������������������ͭ�ijɴ�ͭ����������ͭ�ijɶƼ���
��7���������еĽ���Ĥȥ��������ֻ��H2�ݳ�������д���ҳ��з������ܷ�Ӧ��NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
���� ��1����װ��Ϊȼ�ϵ���ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�����ԭ��أ�Ͷ��ȼ�ϵĵ缫�Ǹ�����������ȼ��ʧ���ӷ���������Ӧ��
��2����װ��Ϊ����Ȼ���װ�ã����缫����ԭ��صĸ�����Ϊ���ص�������ʯīΪ��������������������������
��3����װ��Ϊ����ͭװ�ã�������Zn��Cu��Ag��������п��ͭ��������
��4������ת�Ƶ��������֪���������ܽ��ͭС��������������ͭ�����Ա�װ���з�Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ���С��
��5���ݴ��������ת�Ƶ�������ȵ�������ͭ�Ĺ�ϵʽΪ��O2-----2Cu���㣻
��6����ʯī���缫������Ϊ2L��CuSO4��Һ����ͭ���������������22.2gA��Cu2��OH��2CO3�������ʵ���Ϊ0.1mol������ʹ���Һ�ָ�ԭŨ�������������ͭԪ���غ��֪��n[CuSO4]=0.2mol������c=$\frac{n}{V}$���㣻��������Ƴ��ڶƼ��϶�ͭ��װ�ã���ͭ���������Ƽ���������
��7���������еĽ���Ĥȥ��������ֻ��H2�ݳ��������ɵ��������������Ƽ�����Ӧ�����Ȼ��ƺʹ������ƣ��ݴ���д��
��� �⣺��1����װ��Ϊȼ�ϵ���ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�����ԭ��أ�Ͷ��ȼ�ϵĵ缫�Ǹ����������ϼ���ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ���缫��ӦʽΪ��CH3OCH3-12e-+16OH-�T2CO32-+11H2O��
�ʴ�Ϊ��CH3OCH3-12e-+16OH-�T2CO32-+11H2O��
��2����װ��Ϊ����Ȼ���װ�ã����缫����ԭ��صĸ�����Ϊ���ص�������ʯīΪ�����������������ӷŵ����������������������ӷŵ磬����������������������Ũ�ȴ���������Ũ����Һ�ʼ��ԣ�������װ������������������Ҫ�����������ʴ�Ϊ��������
��3�������ͭ�к���п���������ʣ������ϲ���ͭ����п����ʧ���ӽ�����Һ������������ͭ���ӣ������缫����ʽ�ֱ�ΪZn-2e-=Zn2+��Cu-2e-=Cu2+���ʴ�Ϊ��Zn-2e-=Zn2+��Cu-2e-=Cu2+��
��4���ɣ�3�������缫����ʽ�ֱ�ΪZn-2e-=Zn2+��Cu-2e-=Cu2+������ת�Ƶ��������֪���������ܽ��ͭС��������������ͭ�����Ա�װ���з�Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ���С��
�ʴ�Ϊ����С��
��5�����ݴ��������ת�Ƶ�������ȵ�������ͭ�Ĺ�ϵʽΪ��O2----2Cu��������ͭ��������x��
O2---------2Cu
22.4L 128g
2.24L x
x=12.8g��
�ʴ�Ϊ��12.8 g��
��6����ʯī���缫������Ϊ2L��CuSO4��Һ����ͭ�����������ᣬ���ӷ���ʽΪ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+������22.2gA��Cu2��OH��2CO3�������ʵ���Ϊ0.1mol������ʹ���Һ�ָ�ԭŨ�������������ͭԪ���غ��֪��n[CuSO4]=0.2mol��������ԭ��ҺCuSO4��Ũ����$\frac{0.2mol}{2L}$=0.1mol•L-1����������Ƴ��ڶƼ��϶�ͭ��װ�ã���ͭ���������Ƽ������������Խ�������ͭ�ijɴ�ͭ����������ͭ�ijɶƼ����ɣ��ʴ�Ϊ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+��0.1����������ͭ�ijɴ�ͭ����������ͭ�ijɶƼ���
��7���������еĽ���Ĥȥ��������ֻ��H2�ݳ��������ɵ��������������Ƽ�����Ӧ�����Ȼ��ƺʹ������ƣ��ܷ�ӦΪ��NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2�����ʴ�Ϊ��NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
���� ���⿼��ԭ��غ͵���֪ʶ��Ϊ�߿��������ͣ�������ѧ���ķ��������ͼ��������Ŀ��飬ע����յ缫����ʽ����д��Ϊ������Ĺؼ�����ϴ�����·���ص�����⣬�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��ʹ��̪��Һ������Һ�У�H+��SO42-��Na+��NO3- | |
| B�� | ����Al�ܷų���������Һ�У�NH4+��SO42-��Mg2+��HCO3- | |
| C�� | ���д���Cl2����Һ�У�Ba2+��K+��NO3-��I- | |
| D�� | ǿ���Ե���Һ�У�Cu2+��Br-��Ca2+��Cl- |
| A�� | ��ϩ���Ҵ� | B�� | ��������ϩ | C�� | ����������� | D�� | ��ϩ�;۱�ϩ |
| A�� | 0.1mol•L-1��ˮ�У�c��OH-��=c��NH4+�� | |
| B�� | ��0.1mol•L-1CH3COONa��Һ�У�c��OH-��=c��CH3COOH��+c��H+�� | |
| C�� | 10 mL 0.02mol•L-1HCl��Һ��10 mL 0.02mol•L-1Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH=10 | |
| D�� | 0.1mol•L-1ij��Ԫ����ǿ����NaHA��Һ�У�c��Na+��=2c��A2-��+c��HA-��+c��H2A�� |
| A�� | Na2B��Һ����H2B | |
| B�� | NaHB��Һһ���Լ��� | |
| C�� | NaHB��Һһ�������� | |
| D�� | NaHB��Һ��ϡ���ᷴӦ�����ӷ���ʽ��HB-+H+=H2B |
���ڳ����£���Ļ�ѧ���ʺܻ���
�������������н������ϣ����ɽ����Ȼ���
�۹����ƿ��������Ʊ��轺��ľ�ķ����
��Ư�۾�����Ҫ�ɷ��Ǵ�����ƺ��Ȼ��ƣ�
| A�� | �٢ۢ� | B�� | �٢ڢ� | C�� | �ڢۢ� | D�� | �٢ڢۢ� |
| A�� | 35.5 | B�� | 71g | C�� | 35.5g/mol | D�� | 71g/mol |
| A�� | 11.24 g | B�� | 22.8 g | C�� | 17.02 g | D�� | 24.84 g |